当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of the macrocyclic structure and dynamic solvent effect on the reactivity of a localised singlet diradicaloid with π-single bonding character
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0sc05311b Zhe Wang 1 , Rikuo Akisaka 1 , Sohshi Yabumoto 2 , Tatsuo Nakagawa 2 , Sayaka Hatano 1 , Manabu Abe 1, 3
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0sc05311b Zhe Wang 1 , Rikuo Akisaka 1 , Sohshi Yabumoto 2 , Tatsuo Nakagawa 2 , Sayaka Hatano 1 , Manabu Abe 1, 3
Affiliation
|
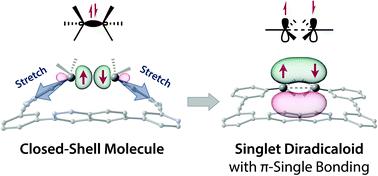
Localised singlet diradicals are key intermediates in bond homolysis processes. Generally, these highly reactive species undergo radical–radical coupling reaction immediately after their generation. Therefore, their short-lived character hampers experimental investigations of their nature. In this study, we implemented the new concept of “stretch effect” to access a kinetically stabilised singlet diradicaloid. To this end, a macrocyclic structure was computationally designed to enable the experimental examination of a singlet diradicaloid with π-single bonding character. The kinetically stabilised diradicaloid exhibited a low carbon–carbon coupling reaction rate of 6.4 × 103 s−1 (155.9 μs), approximately 11 and 1000 times slower than those of the first generation of macrocyclic system (7.0 × 104 s−1, 14.2 μs) and the parent system lacking the macrocycle (5 × 106 s−1, 200 ns) at 293 K in benzene, respectively. In addition, a significant dynamic solvent effect was observed for the first time in intramolecular radical–radical coupling reactions in viscous solvents such as glycerin triacetate. This theoretical and experimental study demonstrates that the stretch effect and solvent viscosity play important roles in retarding the σ-bond formation process, thus enabling a thorough examination of the nature of the singlet diradicaloid and paving the way toward a deeper understanding of reactive intermediates.
中文翻译:

大环结构和动态溶剂效应对具有π-单键特性的局域单线态双自由基反应性的影响
局部单线态双自由基是键均裂过程中的关键中间体。通常,这些高反应性物质在它们产生后立即发生自由基 - 自由基偶联反应。因此,他们短暂的性格阻碍了对其性质的实验研究。在这项研究中,我们实施了“拉伸效应”的新概念来获得动力学稳定的单线态双自由基。为此,计算设计了一个大环结构,以实现对具有 π 单键特性的单线态双自由基的实验检查。动力学稳定的双自由基表现出6.4 × 10 3 s -1的低碳-碳偶联反应速率(155.9 μs),比第一代大环系统 (7.0 × 10 4 s -1 , 14.2 μs) 和缺少大环的母系统 (5 × 10 6 s -1 , 200 ns ) 慢大约 11 和 1000 倍) 在苯中分别为 293 K。此外,在三乙酸甘油酯等粘性溶剂中的分子内自由基-自由基偶联反应中首次观察到显着的动态溶剂效应。这项理论和实验研究表明,拉伸效应和溶剂粘度在延缓 σ 键形成过程中起着重要作用,从而能够彻底检查单线态双自由基的性质,并为更深入地了解活性中间体铺平道路。
更新日期:2020-11-17
中文翻译:

大环结构和动态溶剂效应对具有π-单键特性的局域单线态双自由基反应性的影响
局部单线态双自由基是键均裂过程中的关键中间体。通常,这些高反应性物质在它们产生后立即发生自由基 - 自由基偶联反应。因此,他们短暂的性格阻碍了对其性质的实验研究。在这项研究中,我们实施了“拉伸效应”的新概念来获得动力学稳定的单线态双自由基。为此,计算设计了一个大环结构,以实现对具有 π 单键特性的单线态双自由基的实验检查。动力学稳定的双自由基表现出6.4 × 10 3 s -1的低碳-碳偶联反应速率(155.9 μs),比第一代大环系统 (7.0 × 10 4 s -1 , 14.2 μs) 和缺少大环的母系统 (5 × 10 6 s -1 , 200 ns ) 慢大约 11 和 1000 倍) 在苯中分别为 293 K。此外,在三乙酸甘油酯等粘性溶剂中的分子内自由基-自由基偶联反应中首次观察到显着的动态溶剂效应。这项理论和实验研究表明,拉伸效应和溶剂粘度在延缓 σ 键形成过程中起着重要作用,从而能够彻底检查单线态双自由基的性质,并为更深入地了解活性中间体铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号