当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interactive network of the dehydrogenation of alkanes, alkenes and alkynes – surface carbon hydrogenative coupling on Ru(111)
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0cy02055a Yueyue Jiao 1, 2, 3, 4, 5 , Huan Ma 1, 2, 3, 4, 5 , Hui Wang 1, 2, 3, 4, 5 , Yong-Wang Li 1, 2, 3, 4, 5 , Xiao-Dong Wen 1, 2, 3, 4, 5 , Haijun Jiao 6, 7, 8
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0cy02055a Yueyue Jiao 1, 2, 3, 4, 5 , Huan Ma 1, 2, 3, 4, 5 , Hui Wang 1, 2, 3, 4, 5 , Yong-Wang Li 1, 2, 3, 4, 5 , Xiao-Dong Wen 1, 2, 3, 4, 5 , Haijun Jiao 6, 7, 8
Affiliation

|
To understand the reaction mechanisms of the dehydrogenation and retrosynthesis of alkanes, the consecutive dissociation of methane, ethane, ethene and ethyne, as well as propane, propene and propyne, on the fcc Ru(111) surface has been investigated using periodic density functional theory computations (rPBE). Methane dissociation has the energy minimum path of 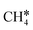 →
→ 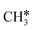 →
→ 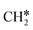 → CH* → C*. Although ethane dissociation does not have ethene and ethyne as intermediates, they have the same final surface species with the minimum energy paths for ethane [
→ CH* → C*. Although ethane dissociation does not have ethene and ethyne as intermediates, they have the same final surface species with the minimum energy paths for ethane [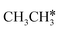 →
→ 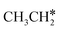 → CH3CH* → CH3C* →
→ CH3CH* → CH3C* → 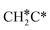 → HC*C* → HC* + C*], ethene [
→ HC*C* → HC* + C*], ethene [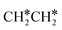 →
→ 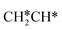 →
→ 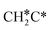 → HC*C* → HC* + C*] and ethyne [CH*CH* → HC*C* → HC* + C*]. Propane dissociation has the competitive routes of n-propyl [
→ HC*C* → HC* + C*] and ethyne [CH*CH* → HC*C* → HC* + C*]. Propane dissociation has the competitive routes of n-propyl [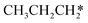 → CH3CH2CH* → CH3CH2C* → CH3CH*C* → CH3C*C* → CH3C* + C* →→ HC* + C*] and isopropyl with propyne as an intermediate [CH3CH*CH3 → CH3C*CH3 →
→ CH3CH2CH* → CH3CH2C* → CH3CH*C* → CH3C*C* → CH3C* + C* →→ HC* + C*] and isopropyl with propyne as an intermediate [CH3CH*CH3 → CH3C*CH3 → 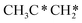 → CH3C*CH* → CH3C*C* →→ HC* + C*], and the n-propyl route has propene as an intermediate for dissociation [
→ CH3C*CH* → CH3C*C* →→ HC* + C*], and the n-propyl route has propene as an intermediate for dissociation [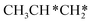 → CH3C*CH2*/CH3CH*CH* → CH3CH*C*/CH3C*CH* → CH3C*C* →→ HC* + C*]. In these reactions, the most stable surface intermediates are HC*, CH3C* and CH3CH2C* as homologs, as found experimentally on other metal surfaces. Our results rationalized the experimentally observed interconversion between
→ CH3C*CH2*/CH3CH*CH* → CH3CH*C*/CH3C*CH* → CH3C*C* →→ HC* + C*]. In these reactions, the most stable surface intermediates are HC*, CH3C* and CH3CH2C* as homologs, as found experimentally on other metal surfaces. Our results rationalized the experimentally observed interconversion between 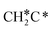 + H* and CH3C* as well as surface HC*C* and CH3C*C* as key intermediates for the first C–C bond dissociation [HC*C* → HC* + C*; CH3C*C* → CH3C* + C*]. On the basis of surface C* and H2 gas, the retrosynthesis of methane, ethane and propane has increasing apparent barriers of 1.08, 1.51 and 1.66 eV, respectively, at 490 K and 1 atm H2 and 0.83, 1.14 and 1.15 eV, respectively, at 19.7 atm H2. Surface carbon coverage changes the formation of alkanes from endergonic to exergonic. This pressure- and coverage-dependency is very important for understanding the reaction mechanism and selectivity. Surface alkynyl groups should be the intermediates for C–C coupling. The computed vibrational frequencies of CH*, CH3C*, CH2C*, HC*C* and
+ H* and CH3C* as well as surface HC*C* and CH3C*C* as key intermediates for the first C–C bond dissociation [HC*C* → HC* + C*; CH3C*C* → CH3C* + C*]. On the basis of surface C* and H2 gas, the retrosynthesis of methane, ethane and propane has increasing apparent barriers of 1.08, 1.51 and 1.66 eV, respectively, at 490 K and 1 atm H2 and 0.83, 1.14 and 1.15 eV, respectively, at 19.7 atm H2. Surface carbon coverage changes the formation of alkanes from endergonic to exergonic. This pressure- and coverage-dependency is very important for understanding the reaction mechanism and selectivity. Surface alkynyl groups should be the intermediates for C–C coupling. The computed vibrational frequencies of CH*, CH3C*, CH2C*, HC*C* and 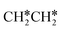 agree with the experiments. The comparison in adsorption energies and reaction barriers and energies shows that the fcc Ru(111) surface is more active than the hcp Ru(0001) surface despite their very similar surface structures.
agree with the experiments. The comparison in adsorption energies and reaction barriers and energies shows that the fcc Ru(111) surface is more active than the hcp Ru(0001) surface despite their very similar surface structures.
中文翻译:

烷烃,烯烃和炔烃脱氢的相互作用网络-Ru(111)上的表面碳氢化偶联
为了解烷烃脱氢和反合成的反应机理,使用周期密度泛函理论研究了甲烷,乙烷,乙烯和乙炔以及丙烷,丙烯和丙炔在fcc Ru(111)表面上的连续解离计算(rPBE)。甲烷离解具有的能量最小路径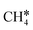 →
→ 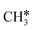 →
→ 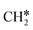 →CH *→C *。虽然乙烷解离不具有乙烯和乙炔作为中间体,它们具有与乙烷[最小能量路径相同的最终表面物种
→CH *→C *。虽然乙烷解离不具有乙烯和乙炔作为中间体,它们具有与乙烷[最小能量路径相同的最终表面物种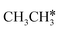 →
→ 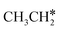 →CH 3 CH *→CH 3 C *→
→CH 3 CH *→CH 3 C *→ 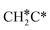 →HC * C *→HC * + C * ],乙烯[
→HC * C *→HC * + C * ],乙烯[ 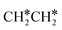 →交通
→交通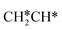 →交通
→交通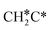 →HC * C *→HC * + C *]和乙炔[CH * CH *→HC * C *→HC * + C *]。丙烷离解具有正丙基的竞争途径[
→HC * C *→HC * + C *]和乙炔[CH * CH *→HC * C *→HC * + C *]。丙烷离解具有正丙基的竞争途径[ 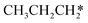 →CH 3 CH 2 CH *→CH 3 CH 2 C *→CH 3 CH * C *→CH 3 C * C *→CH 3 C * + C *→→HC * + C *]和异丙基用丙炔作为中间体[CH 3 CH * CH 3 →CH 3 C * CH 3 →
→CH 3 CH 2 CH *→CH 3 CH 2 C *→CH 3 CH * C *→CH 3 C * C *→CH 3 C * + C *→→HC * + C *]和异丙基用丙炔作为中间体[CH 3 CH * CH 3 →CH 3 C * CH 3 → 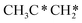 →CH 3 C * CH *→CH 3 C * C *→→HC * + C *],并且正丙基路线以丙烯为离解中间体[
→CH 3 C * CH *→CH 3 C * C *→→HC * + C *],并且正丙基路线以丙烯为离解中间体[ 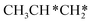 →CH 3 C * CH2 * / CH 3 CH * CH *→CH 3 CH * C * / CH 3 C * CH *→CH 3 C * C *→→HC * + C *]。在这些反应中,最稳定的表面中间体是HC *,CH 3 C *和CH 3 CH 2 C *作为同系物,如在其他金属表面上的实验发现。我们的结果合理化了在实验中观察到的
→CH 3 C * CH2 * / CH 3 CH * CH *→CH 3 CH * C * / CH 3 C * CH *→CH 3 C * C *→→HC * + C *]。在这些反应中,最稳定的表面中间体是HC *,CH 3 C *和CH 3 CH 2 C *作为同系物,如在其他金属表面上的实验发现。我们的结果合理化了在实验中观察到的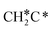 + H *和CH 3 C *以及表面HC * C *和CH 3 C * C *之间的相互转化,这是第一次C–C键解离的关键中间体[HC * C *→HC * + C *; CH 3 C * C *→CH 3 C * + C *]。基于表面C *和H 2气体中,甲烷,乙烷和丙烷的逆合成在490 K和1 atm H 2下分别具有1.08、1.51和1.66 eV的表观势垒,在19.7 atm H 2下分别具有0.83、1.14和1.15 eV的表观势垒。表面碳的覆盖范围将烷烃的形成从二十碳五烯转变为十二碳。这种压力和覆盖率依赖性对于理解反应机理和选择性非常重要。表面炔基应该是CC偶联的中间体。计算的CH *,CH 3 C *,CH 2 C *,HC * C *和
+ H *和CH 3 C *以及表面HC * C *和CH 3 C * C *之间的相互转化,这是第一次C–C键解离的关键中间体[HC * C *→HC * + C *; CH 3 C * C *→CH 3 C * + C *]。基于表面C *和H 2气体中,甲烷,乙烷和丙烷的逆合成在490 K和1 atm H 2下分别具有1.08、1.51和1.66 eV的表观势垒,在19.7 atm H 2下分别具有0.83、1.14和1.15 eV的表观势垒。表面碳的覆盖范围将烷烃的形成从二十碳五烯转变为十二碳。这种压力和覆盖率依赖性对于理解反应机理和选择性非常重要。表面炔基应该是CC偶联的中间体。计算的CH *,CH 3 C *,CH 2 C *,HC * C *和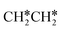 同意实验。吸附能,反应势垒能和吸附能的比较表明,尽管fcc Ru(111)表面非常相似,但其表面活性却比hcp Ru(0001)表面高。
同意实验。吸附能,反应势垒能和吸附能的比较表明,尽管fcc Ru(111)表面非常相似,但其表面活性却比hcp Ru(0001)表面高。
更新日期:2020-11-17
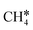 →
→ 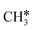 →
→ 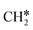 → CH* → C*. Although ethane dissociation does not have ethene and ethyne as intermediates, they have the same final surface species with the minimum energy paths for ethane [
→ CH* → C*. Although ethane dissociation does not have ethene and ethyne as intermediates, they have the same final surface species with the minimum energy paths for ethane [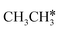 →
→ 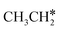 → CH3CH* → CH3C* →
→ CH3CH* → CH3C* → 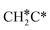 → HC*C* → HC* + C*], ethene [
→ HC*C* → HC* + C*], ethene [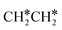 →
→ 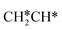 →
→ 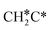 → HC*C* → HC* + C*] and ethyne [CH*CH* → HC*C* → HC* + C*]. Propane dissociation has the competitive routes of n-propyl [
→ HC*C* → HC* + C*] and ethyne [CH*CH* → HC*C* → HC* + C*]. Propane dissociation has the competitive routes of n-propyl [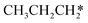 → CH3CH2CH* → CH3CH2C* → CH3CH*C* → CH3C*C* → CH3C* + C* →→ HC* + C*] and isopropyl with propyne as an intermediate [CH3CH*CH3 → CH3C*CH3 →
→ CH3CH2CH* → CH3CH2C* → CH3CH*C* → CH3C*C* → CH3C* + C* →→ HC* + C*] and isopropyl with propyne as an intermediate [CH3CH*CH3 → CH3C*CH3 → 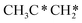 → CH3C*CH* → CH3C*C* →→ HC* + C*], and the n-propyl route has propene as an intermediate for dissociation [
→ CH3C*CH* → CH3C*C* →→ HC* + C*], and the n-propyl route has propene as an intermediate for dissociation [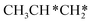 → CH3C*CH2*/CH3CH*CH* → CH3CH*C*/CH3C*CH* → CH3C*C* →→ HC* + C*]. In these reactions, the most stable surface intermediates are HC*, CH3C* and CH3CH2C* as homologs, as found experimentally on other metal surfaces. Our results rationalized the experimentally observed interconversion between
→ CH3C*CH2*/CH3CH*CH* → CH3CH*C*/CH3C*CH* → CH3C*C* →→ HC* + C*]. In these reactions, the most stable surface intermediates are HC*, CH3C* and CH3CH2C* as homologs, as found experimentally on other metal surfaces. Our results rationalized the experimentally observed interconversion between 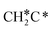 + H* and CH3C* as well as surface HC*C* and CH3C*C* as key intermediates for the first C–C bond dissociation [HC*C* → HC* + C*; CH3C*C* → CH3C* + C*]. On the basis of surface C* and H2 gas, the retrosynthesis of methane, ethane and propane has increasing apparent barriers of 1.08, 1.51 and 1.66 eV, respectively, at 490 K and 1 atm H2 and 0.83, 1.14 and 1.15 eV, respectively, at 19.7 atm H2. Surface carbon coverage changes the formation of alkanes from endergonic to exergonic. This pressure- and coverage-dependency is very important for understanding the reaction mechanism and selectivity. Surface alkynyl groups should be the intermediates for C–C coupling. The computed vibrational frequencies of CH*, CH3C*, CH2C*, HC*C* and
+ H* and CH3C* as well as surface HC*C* and CH3C*C* as key intermediates for the first C–C bond dissociation [HC*C* → HC* + C*; CH3C*C* → CH3C* + C*]. On the basis of surface C* and H2 gas, the retrosynthesis of methane, ethane and propane has increasing apparent barriers of 1.08, 1.51 and 1.66 eV, respectively, at 490 K and 1 atm H2 and 0.83, 1.14 and 1.15 eV, respectively, at 19.7 atm H2. Surface carbon coverage changes the formation of alkanes from endergonic to exergonic. This pressure- and coverage-dependency is very important for understanding the reaction mechanism and selectivity. Surface alkynyl groups should be the intermediates for C–C coupling. The computed vibrational frequencies of CH*, CH3C*, CH2C*, HC*C* and 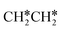 agree with the experiments. The comparison in adsorption energies and reaction barriers and energies shows that the fcc Ru(111) surface is more active than the hcp Ru(0001) surface despite their very similar surface structures.
agree with the experiments. The comparison in adsorption energies and reaction barriers and energies shows that the fcc Ru(111) surface is more active than the hcp Ru(0001) surface despite their very similar surface structures.
中文翻译:

烷烃,烯烃和炔烃脱氢的相互作用网络-Ru(111)上的表面碳氢化偶联
为了解烷烃脱氢和反合成的反应机理,使用周期密度泛函理论研究了甲烷,乙烷,乙烯和乙炔以及丙烷,丙烯和丙炔在fcc Ru(111)表面上的连续解离计算(rPBE)。甲烷离解具有的能量最小路径
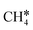 →
→ 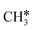 →
→ 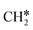 →CH *→C *。虽然乙烷解离不具有乙烯和乙炔作为中间体,它们具有与乙烷[最小能量路径相同的最终表面物种
→CH *→C *。虽然乙烷解离不具有乙烯和乙炔作为中间体,它们具有与乙烷[最小能量路径相同的最终表面物种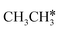 →
→ 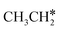 →CH 3 CH *→CH 3 C *→
→CH 3 CH *→CH 3 C *→ 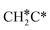 →HC * C *→HC * + C * ],乙烯[
→HC * C *→HC * + C * ],乙烯[ 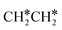 →交通
→交通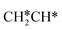 →交通
→交通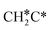 →HC * C *→HC * + C *]和乙炔[CH * CH *→HC * C *→HC * + C *]。丙烷离解具有正丙基的竞争途径[
→HC * C *→HC * + C *]和乙炔[CH * CH *→HC * C *→HC * + C *]。丙烷离解具有正丙基的竞争途径[ 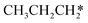 →CH 3 CH 2 CH *→CH 3 CH 2 C *→CH 3 CH * C *→CH 3 C * C *→CH 3 C * + C *→→HC * + C *]和异丙基用丙炔作为中间体[CH 3 CH * CH 3 →CH 3 C * CH 3 →
→CH 3 CH 2 CH *→CH 3 CH 2 C *→CH 3 CH * C *→CH 3 C * C *→CH 3 C * + C *→→HC * + C *]和异丙基用丙炔作为中间体[CH 3 CH * CH 3 →CH 3 C * CH 3 → 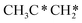 →CH 3 C * CH *→CH 3 C * C *→→HC * + C *],并且正丙基路线以丙烯为离解中间体[
→CH 3 C * CH *→CH 3 C * C *→→HC * + C *],并且正丙基路线以丙烯为离解中间体[ 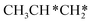 →CH 3 C * CH2 * / CH 3 CH * CH *→CH 3 CH * C * / CH 3 C * CH *→CH 3 C * C *→→HC * + C *]。在这些反应中,最稳定的表面中间体是HC *,CH 3 C *和CH 3 CH 2 C *作为同系物,如在其他金属表面上的实验发现。我们的结果合理化了在实验中观察到的
→CH 3 C * CH2 * / CH 3 CH * CH *→CH 3 CH * C * / CH 3 C * CH *→CH 3 C * C *→→HC * + C *]。在这些反应中,最稳定的表面中间体是HC *,CH 3 C *和CH 3 CH 2 C *作为同系物,如在其他金属表面上的实验发现。我们的结果合理化了在实验中观察到的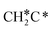 + H *和CH 3 C *以及表面HC * C *和CH 3 C * C *之间的相互转化,这是第一次C–C键解离的关键中间体[HC * C *→HC * + C *; CH 3 C * C *→CH 3 C * + C *]。基于表面C *和H 2气体中,甲烷,乙烷和丙烷的逆合成在490 K和1 atm H 2下分别具有1.08、1.51和1.66 eV的表观势垒,在19.7 atm H 2下分别具有0.83、1.14和1.15 eV的表观势垒。表面碳的覆盖范围将烷烃的形成从二十碳五烯转变为十二碳。这种压力和覆盖率依赖性对于理解反应机理和选择性非常重要。表面炔基应该是CC偶联的中间体。计算的CH *,CH 3 C *,CH 2 C *,HC * C *和
+ H *和CH 3 C *以及表面HC * C *和CH 3 C * C *之间的相互转化,这是第一次C–C键解离的关键中间体[HC * C *→HC * + C *; CH 3 C * C *→CH 3 C * + C *]。基于表面C *和H 2气体中,甲烷,乙烷和丙烷的逆合成在490 K和1 atm H 2下分别具有1.08、1.51和1.66 eV的表观势垒,在19.7 atm H 2下分别具有0.83、1.14和1.15 eV的表观势垒。表面碳的覆盖范围将烷烃的形成从二十碳五烯转变为十二碳。这种压力和覆盖率依赖性对于理解反应机理和选择性非常重要。表面炔基应该是CC偶联的中间体。计算的CH *,CH 3 C *,CH 2 C *,HC * C *和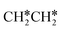 同意实验。吸附能,反应势垒能和吸附能的比较表明,尽管fcc Ru(111)表面非常相似,但其表面活性却比hcp Ru(0001)表面高。
同意实验。吸附能,反应势垒能和吸附能的比较表明,尽管fcc Ru(111)表面非常相似,但其表面活性却比hcp Ru(0001)表面高。




























 京公网安备 11010802027423号
京公网安备 11010802027423号