当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methanol synthesis over Cu/CeO2–ZrO2 catalysts: the key role of multiple active components
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0cy01762k Maxim Zabilskiy 1, 2, 3, 4 , Kaibo Ma 1, 2, 3, 4 , Arik Beck 1, 2, 3, 4, 5 , Jeroen A. van Bokhoven 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0cy01762k Maxim Zabilskiy 1, 2, 3, 4 , Kaibo Ma 1, 2, 3, 4 , Arik Beck 1, 2, 3, 4, 5 , Jeroen A. van Bokhoven 1, 2, 3, 4, 5
Affiliation
|
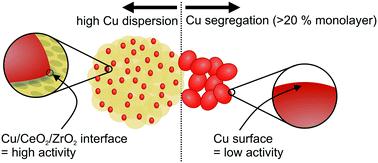
High surface area ceria–zirconia synthesized by a glycothermal approach was used as a support for copper nanoparticles. Cu–CeO2/ZrO2 catalysts containing 5–25 wt% copper demonstrate high carbon dioxide-to-methanol conversion rates (120–180 gMeOH kgcat−1 h−1) at 260 °C and 50 bar. The sample containing 5 wt% copper in the form of small nanoparticles (≤5 nm) demonstrates the highest activity normalized per mass of copper, while higher copper loading results in copper segregation and correspondingly lower activity. We attribute the high activity to a unique synergetic effect between the active components, copper, ceria and zirconia, where activation of hydrogen and carbon dioxide and subsequent methanol synthesis take place. The redox properties of the ceria–zirconia support and its ability to form oxygen vacancy sites play a crucial role in carbon dioxide activation.
中文翻译:

Cu / CeO2-ZrO2催化剂上的甲醇合成:多种活性成分的关键作用
通过糖热法合成的高表面积二氧化铈-氧化锆用作铜纳米粒子的载体。含有5–25 wt%的铜的Cu–CeO 2 / ZrO 2催化剂显示出高的二氧化碳转化为甲醇的转化率(120–180 g MeOH kg cat -1 h -1)在260°C和50 bar下。包含5 wt%的小纳米颗粒(≤5nm)形式的铜的样品表现出最高的活性/单位质量铜,而较高的铜负载量导致铜偏析和相应较低的活性。我们将高活性归因于活性成分铜,二氧化铈和氧化锆之间的独特协同作用,其中氢和二氧化碳的活化以及随后的甲醇合成发生。氧化铈-氧化锆载体的氧化还原特性及其形成氧空位的能力在二氧化碳活化中起着至关重要的作用。
更新日期:2020-11-17
中文翻译:

Cu / CeO2-ZrO2催化剂上的甲醇合成:多种活性成分的关键作用
通过糖热法合成的高表面积二氧化铈-氧化锆用作铜纳米粒子的载体。含有5–25 wt%的铜的Cu–CeO 2 / ZrO 2催化剂显示出高的二氧化碳转化为甲醇的转化率(120–180 g MeOH kg cat -1 h -1)在260°C和50 bar下。包含5 wt%的小纳米颗粒(≤5nm)形式的铜的样品表现出最高的活性/单位质量铜,而较高的铜负载量导致铜偏析和相应较低的活性。我们将高活性归因于活性成分铜,二氧化铈和氧化锆之间的独特协同作用,其中氢和二氧化碳的活化以及随后的甲醇合成发生。氧化铈-氧化锆载体的氧化还原特性及其形成氧空位的能力在二氧化碳活化中起着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号