当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stability of Human Serum Amyloid A Fibrils
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jpcb.0c08280 Wenhua Wang 1 , Ulrich H E Hansmann 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jpcb.0c08280 Wenhua Wang 1 , Ulrich H E Hansmann 1
Affiliation
|
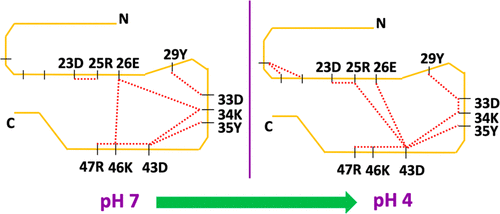
In systemic amyloidosis, serum amyloid A (SAA) fibril deposits cause widespread damages to tissues and organs that eventually may lead to death. A therapeutically intervention therefore has either to dissolve these fibrils or inhibit their formation. However, only recently has the human SAA fibril structure been resolved at a resolution that is sufficient for development of drug candidates. Here, we use molecular dynamic simulations to probe the factors that modulate the stability of this fibril model. Our simulations suggest that fibril formation starts with the stacking of two misfolded monomers into metastable dimers, with the stacking depending on the N-terminal amyloidogenic regions of different chains forming anchors. The resulting dimers pack in a second step into a 2-fold two-layer tetramer that is stable enough to nucleate fibril formation. The stability of the initial dimers is enhanced under acidic conditions by a strong salt bridge and side-chain hydrogen bond network in the C-terminal cavity (residues 23–51) but is not affected by the presence of the disordered C-terminal tail.
中文翻译:

人血清淀粉样蛋白 A 原纤维的稳定性
在系统性淀粉样变性中,血清淀粉样蛋白 A (SAA) 原纤维沉积物对组织和器官造成广泛损害,最终可能导致死亡。因此,治疗干预必须溶解这些原纤维或抑制它们的形成。然而,直到最近才以足以开发候选药物的分辨率解析人类 SAA 原纤维结构。在这里,我们使用分子动力学模拟来探索调节该原纤维模型稳定性的因素。我们的模拟表明,原纤维的形成始于将两个错误折叠的单体堆叠成亚稳态二聚体,堆叠取决于形成锚的不同链的 N 端淀粉样蛋白生成区域。所得二聚体在第二步中组装成 2 倍双层四聚体,该四聚体足够稳定以形成原纤维成核。初始二聚体的稳定性在酸性条件下通过 C 端空腔(残基 23-51)中的强盐桥和侧链氢键网络增强,但不受无序 C 端尾部的影响。
更新日期:2020-11-25
中文翻译:

人血清淀粉样蛋白 A 原纤维的稳定性
在系统性淀粉样变性中,血清淀粉样蛋白 A (SAA) 原纤维沉积物对组织和器官造成广泛损害,最终可能导致死亡。因此,治疗干预必须溶解这些原纤维或抑制它们的形成。然而,直到最近才以足以开发候选药物的分辨率解析人类 SAA 原纤维结构。在这里,我们使用分子动力学模拟来探索调节该原纤维模型稳定性的因素。我们的模拟表明,原纤维的形成始于将两个错误折叠的单体堆叠成亚稳态二聚体,堆叠取决于形成锚的不同链的 N 端淀粉样蛋白生成区域。所得二聚体在第二步中组装成 2 倍双层四聚体,该四聚体足够稳定以形成原纤维成核。初始二聚体的稳定性在酸性条件下通过 C 端空腔(残基 23-51)中的强盐桥和侧链氢键网络增强,但不受无序 C 端尾部的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号