当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the Allosteric Pathway That Interconnects the Core-Functional Scaffold and the Distal Phosphorylation Sites for Specific Dephosphorylation of Bcl-2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jmedchem.0c01290 Ziqian Wang 1 , Ting Song 1 , Zongwei Guo 2 , Keke Cao 2 , Chao Chen 3 , Yingang Feng 3 , Hang Wang 4 , Fangkui Yin 1 , Sheng Zhou 1 , Jian Dai 1 , Zhichao Zhang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jmedchem.0c01290 Ziqian Wang 1 , Ting Song 1 , Zongwei Guo 2 , Keke Cao 2 , Chao Chen 3 , Yingang Feng 3 , Hang Wang 4 , Fangkui Yin 1 , Sheng Zhou 1 , Jian Dai 1 , Zhichao Zhang 1
Affiliation
|
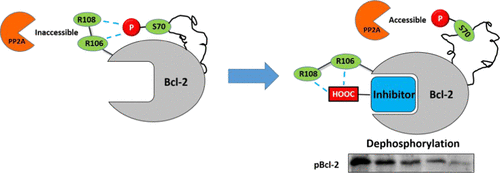
Protein phosphorylation is the most significant post-translational modification for regulating cellular activities, but site-specific modulation of phosphorylation is still challenging. Using three-dimensional NMR spectra, molecular dynamics simulations, and alanine mutations, we identified that the interaction network between pT69/pS70 and R106/R109 residues prevents the phosphorylation sites from exposure to phosphatase and subsequent dephosphorylation. A Bcl-2-dephosphorylation probe, S1-6e, was designed by installing a carboxylic acid group to a Bcl-2 inhibitor. The carboxyl group competitively disrupts the interaction network between R106/R109 and pT69/pS70 and subsequently facilitates Bcl-2 dephosphorylation in living cells. As a result, S1-6e manifests a more effective apoptosis induction in pBcl-2-dependent cancer cells than other inhibitors exhibiting a similar binding affinity for Bcl-2. We believe that targeting the allosteric pathways interconnecting the core-functional domain and the phosphorylation site can be a general strategy for a rational design of site-specific dephosphorylating probes, since the allosteric pathway has been discovered in a variety of proteins.
中文翻译:

针对互连核心功能支架和远端磷酸化位点的Bcl-2特异性磷酸化的变构途径。
蛋白质磷酸化是调节细胞活性的最重要的翻译后修饰,但是磷酸化的位点特异性调节仍然具有挑战性。使用三维核磁共振光谱,分子动力学模拟和丙氨酸突变,我们发现pT69 / pS70和R106 / R109残基之间的相互作用网络防止磷酸化位点暴露于磷酸酶和随后的去磷酸化。通过将羧酸基团安装到Bcl-2抑制剂上来设计Bcl-2-脱磷酸探针S1-6e。羧基竞争性破坏R106 / R109和pT69 / pS70之间的相互作用网络,并随后促进活细胞中Bcl-2的去磷酸化。结果,S1-6e与对Bcl-2表现出相似结合亲和力的其他抑制剂相比,在pBcl-2依赖性癌细胞中更有效的诱导凋亡诱导作用。我们认为,靶向互变构途径将核心功能结构域和磷酸化位点互连可以是合理设计位点特异性去磷酸化探针的一般策略,因为已在多种蛋白质中发现了变构途径。
更新日期:2020-11-25
中文翻译:

针对互连核心功能支架和远端磷酸化位点的Bcl-2特异性磷酸化的变构途径。
蛋白质磷酸化是调节细胞活性的最重要的翻译后修饰,但是磷酸化的位点特异性调节仍然具有挑战性。使用三维核磁共振光谱,分子动力学模拟和丙氨酸突变,我们发现pT69 / pS70和R106 / R109残基之间的相互作用网络防止磷酸化位点暴露于磷酸酶和随后的去磷酸化。通过将羧酸基团安装到Bcl-2抑制剂上来设计Bcl-2-脱磷酸探针S1-6e。羧基竞争性破坏R106 / R109和pT69 / pS70之间的相互作用网络,并随后促进活细胞中Bcl-2的去磷酸化。结果,S1-6e与对Bcl-2表现出相似结合亲和力的其他抑制剂相比,在pBcl-2依赖性癌细胞中更有效的诱导凋亡诱导作用。我们认为,靶向互变构途径将核心功能结构域和磷酸化位点互连可以是合理设计位点特异性去磷酸化探针的一般策略,因为已在多种蛋白质中发现了变构途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号