当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Volumetric and Viscometric Properties of Maltitol in Glycylglycine Aqueous Solutions at T = 293.15–333.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.jced.0c00731 Yunlong Shi 1 , Chunying Zhu 1 , Taotao Fu 1 , Xiqun Gao 2 , Youguang Ma 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-17 , DOI: 10.1021/acs.jced.0c00731 Yunlong Shi 1 , Chunying Zhu 1 , Taotao Fu 1 , Xiqun Gao 2 , Youguang Ma 1
Affiliation
|
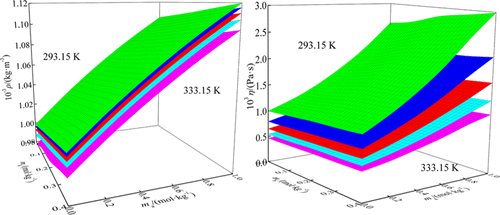
The densities and viscosities of maltitol in glycylglycine aqueous solutions were measured at T = 293.15, 303.15, 313.15, 323.15, and 333.15 K and at atmospheric pressure. Furthermore, the apparent molar volume (Vφ), the limiting partial molar volume (Vφ0), the limiting partial molar volume of transfer (ΔtrVφ0), the viscosity B coefficient, and the active free energies per mole of the solvent and solute (Δμ10≠ and Δμ20≠) were obtained from the experimental densities and viscosities. Moreover, the limiting partial molar volume of transfer was analyzed based on the cosphere overlap model, and the active free energies were also discussed according to the transition-state theory. The results indicate that the ion–hydrophilic interaction between the maltitol and glycylglycine molecules occupies a dominant position in the solution, and maltitol tends to act as a structural maker in the glycylglycine aqueous solution.
中文翻译:

糖基甘氨酸水溶液中麦芽糖醇在T = 293.15–333.15 K时的体积和粘度特性
在T = 293.15、303.15、313.15、323.15和333.15 K且在大气压下测量甘氨酰甘氨酸水溶液中麦芽糖醇的密度和粘度。此外,表观摩尔体积(V φ),所述限制部分摩尔体积(V φ 0),转移的极限偏摩尔体积(Δ TR V φ 0),粘度乙系数,和每摩尔的活性自由能溶剂和溶质(Δμ 1 0≠和Δμ 2 0≠)是从实验密度和粘度获得的。此外,在共球交叠模型的基础上,分析了有限的部分摩尔转移量,并根据过渡态理论讨论了活性自由能。结果表明,麦芽糖醇和甘氨酰甘氨酸分子之间的离子-亲水相互作用在溶液中占主导地位,麦芽糖醇倾向于在甘氨酰甘氨酸水溶液中起结构制造者的作用。
更新日期:2021-01-14
中文翻译:

糖基甘氨酸水溶液中麦芽糖醇在T = 293.15–333.15 K时的体积和粘度特性
在T = 293.15、303.15、313.15、323.15和333.15 K且在大气压下测量甘氨酰甘氨酸水溶液中麦芽糖醇的密度和粘度。此外,表观摩尔体积(V φ),所述限制部分摩尔体积(V φ 0),转移的极限偏摩尔体积(Δ TR V φ 0),粘度乙系数,和每摩尔的活性自由能溶剂和溶质(Δμ 1 0≠和Δμ 2 0≠)是从实验密度和粘度获得的。此外,在共球交叠模型的基础上,分析了有限的部分摩尔转移量,并根据过渡态理论讨论了活性自由能。结果表明,麦芽糖醇和甘氨酰甘氨酸分子之间的离子-亲水相互作用在溶液中占主导地位,麦芽糖醇倾向于在甘氨酰甘氨酸水溶液中起结构制造者的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号