当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rates and Dynamics of Mercury Isotope Exchange between Dissolved Elemental Hg(0) and Hg(II) Bound to Organic and Inorganic Ligands
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.est.0c06229 Quanying Wang 1, 2 , Lijie Zhang 2 , Xujun Liang 2 , Xiangping Yin 2 , Yaoling Zhang 2 , Wang Zheng 2, 3 , Eric M. Pierce 2 , Baohua Gu 2, 4
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.est.0c06229 Quanying Wang 1, 2 , Lijie Zhang 2 , Xujun Liang 2 , Xiangping Yin 2 , Yaoling Zhang 2 , Wang Zheng 2, 3 , Eric M. Pierce 2 , Baohua Gu 2, 4
Affiliation
|
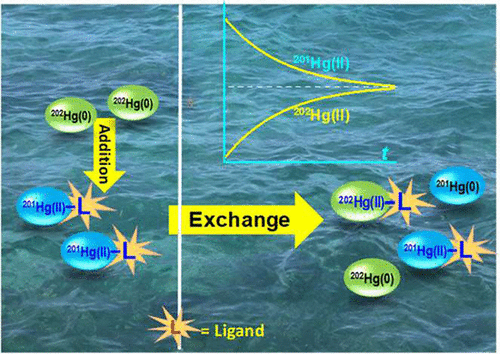
Mercury (Hg) isotope exchange is a common process in biogeochemical transformations of Hg in the environment, but it is unclear whether and at what rates dissolved elemental Hg(0)aq may exchange with divalent Hg(II) bound to various organic and inorganic ligands in water. Using enriched stable isotopes, we investigated the rates and dynamics of isotope exchange between 202Hg(0)aq and 201Hg(II) bound to organic and inorganic ligands with varying chemical structures and binding affinities. Time-dependent exchange reactions were followed by isotope compositional changes using both inductively coupled plasma mass spectrometry and Zeeman cold vapor atomic absorption spectrometry. Rapid, spontaneous isotope exchange (<1 h) was observed between 202Hg(0)aq and 201Hg(II) bound to chloride (Cl–), ethylenediaminetetraacetate (EDTA), and thiols, such as cysteine (CYS), glutathione (GSH), and 2,3-dimercaptopropanesulfonic acid (DMPS) at a thiol ligand-to-Hg(II) molar ratio of 1:1. Without external reductants or oxidants, the exchange resulted in transfer of two electrons and redistribution of Hg isotopes bound to the ligand but no net changes of chemical species in the system. However, an increase in the ligand-to-Hg(II) ratio decreased the exchange rates due to the formation of 2:1 or higher thiol:Hg(II) chelated complexes, but had no effects on exchange rates with 201Hg(II) bound to EDTA or Cl–. The exchange between 202Hg(0)aq and 201Hg(II) bound to dissolved organic matter (DOM) showed an initially rapid followed by a slower exchange rate, likely resulting from Hg(II) complexation with both low- and high-affinity binding functional groups on DOM (e.g., carboxylates vs bidentate thiolates). These results demonstrate that Hg(0)aq readily exchanges with Hg(II) bound to various ligands and highlight the importance of considering exchange reactions in experimental enriched Hg isotope tracer studies or in natural abundance Hg isotope studies in environmental matrices.
中文翻译:

溶解于有机和无机配体中的元素Hg(0)和Hg(II)中的汞同位素交换速率和动力学
汞(Hg)同位素交换是环境中汞的生物地球化学转化中的常见过程,但尚不清楚溶解的元素Hg(0)水溶液是否以及以何种速率与结合到各种有机和无机配体上的二价Hg(II)交换在水里。使用富集的稳定同位素,我们研究了202 Hg(0)aq和201之间的同位素交换速率和动力学Hg(II)以不同的化学结构和结合亲和力与有机和无机配体结合。时间依赖性交换反应之后,使用电感耦合等离子体质谱法和塞曼冷蒸气原子吸收光谱法进行同位素组成变化。观察到202 Hg(0)水溶液和201 Hg(II)结合氯化物(Cl –),乙二胺四乙酸盐(EDTA)和硫醇,例如半胱氨酸(CYS),谷胱甘肽(GSH)和2,3-二巯基丙烷磺酸(DMPS),硫醇配体与Hg(II)的摩尔比为1:1。在没有外部还原剂或氧化剂的情况下,交换导致两个电子的转移以及与配体结合的Hg同位素的重新分布,但系统中化学物种没有净变化。但是,配体与Hg(II)比率的增加由于形成2:1或更高的硫醇:Hg(II)螯合复合物而降低了交换速率,但对201 Hg(II)的交换速率没有影响)绑定到EDTA或Cl –。202 Hg(0)aq与201之间的交换与溶解有机物(DOM)结合的Hg(II)最初显示很快,随后是较慢的交换速率,这很可能是由于Hg(II)与DOM上的低亲和力和高亲和力结合官能团(例如羧酸盐对双齿酸盐)络合所致硫醇盐)。这些结果表明,Hg(0)水溶液易于与结合到各种配体上的Hg(II)交换,并突出了在实验富集的Hg同位素示踪剂研究或环境基质中自然丰度Hg同位素研究中考虑交换反应的重要性。
更新日期:2020-12-01
中文翻译:

溶解于有机和无机配体中的元素Hg(0)和Hg(II)中的汞同位素交换速率和动力学
汞(Hg)同位素交换是环境中汞的生物地球化学转化中的常见过程,但尚不清楚溶解的元素Hg(0)水溶液是否以及以何种速率与结合到各种有机和无机配体上的二价Hg(II)交换在水里。使用富集的稳定同位素,我们研究了202 Hg(0)aq和201之间的同位素交换速率和动力学Hg(II)以不同的化学结构和结合亲和力与有机和无机配体结合。时间依赖性交换反应之后,使用电感耦合等离子体质谱法和塞曼冷蒸气原子吸收光谱法进行同位素组成变化。观察到202 Hg(0)水溶液和201 Hg(II)结合氯化物(Cl –),乙二胺四乙酸盐(EDTA)和硫醇,例如半胱氨酸(CYS),谷胱甘肽(GSH)和2,3-二巯基丙烷磺酸(DMPS),硫醇配体与Hg(II)的摩尔比为1:1。在没有外部还原剂或氧化剂的情况下,交换导致两个电子的转移以及与配体结合的Hg同位素的重新分布,但系统中化学物种没有净变化。但是,配体与Hg(II)比率的增加由于形成2:1或更高的硫醇:Hg(II)螯合复合物而降低了交换速率,但对201 Hg(II)的交换速率没有影响)绑定到EDTA或Cl –。202 Hg(0)aq与201之间的交换与溶解有机物(DOM)结合的Hg(II)最初显示很快,随后是较慢的交换速率,这很可能是由于Hg(II)与DOM上的低亲和力和高亲和力结合官能团(例如羧酸盐对双齿酸盐)络合所致硫醇盐)。这些结果表明,Hg(0)水溶液易于与结合到各种配体上的Hg(II)交换,并突出了在实验富集的Hg同位素示踪剂研究或环境基质中自然丰度Hg同位素研究中考虑交换反应的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号