当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metabolic Profiles of Tetrabromobisphenol A in Humans Extrapolated from Humanized-Liver Mouse Data Using a Simplified Physiologically Based Pharmacokinetic Model
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.chemrestox.0c00358 Tomonori Miura 1 , Shotaro Uehara 2 , Kazuki Shigeta 1 , Manae Yoshizawa 1 , Yusuke Kamiya 1 , Norie Murayama 1 , Makiko Shimizu 1 , Hiroshi Suemizu 2 , Hiroshi Yamazaki 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.chemrestox.0c00358 Tomonori Miura 1 , Shotaro Uehara 2 , Kazuki Shigeta 1 , Manae Yoshizawa 1 , Yusuke Kamiya 1 , Norie Murayama 1 , Makiko Shimizu 1 , Hiroshi Suemizu 2 , Hiroshi Yamazaki 1
Affiliation
|
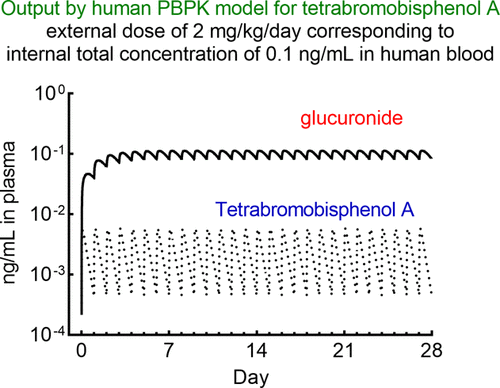
Tetrabromobisphenol A, a brominated flame retardant, is increasingly prevalent worldwide and presents a potential health risk. Adjusted animal biomonitoring equivalents of tetrabromobisphenol A after orally administered doses in humanized-liver mice were scaled up to humans using known species allometric scaling factors to set up simplified physiologically based pharmacokinetic (PBPK) models. Absorbed tetrabromobisphenol A was slightly, moderately, and extensively metabolized in vivo to its glucuronide in rats, control mice, and humanized-liver mice tested, respectively. In silico estimated hepatic exposures of tetrabromobisphenol A and its glucuronide generated using the rat PBPK model-generated plasma concentration profiles were consistent with the reported values. The extent of hepatic injury in humanized-liver mice caused by tetrabromobisphenol A was evaluated by detecting human albumin mRNA in mouse plasma after oral administration of a high dose of tetrabromobisphenol A (1000 mg/kg). Reverse dosimetry analyses were carried out using two human PBPK models (set up based on the humanized-liver-mouse model and by optimizing the input parameters for reported human plasma concentrations of tetrabromobisphenol A glucuronide) to estimate the tetrabromobisphenol A daily intake based on reported human serum concentrations of total tetrabromobisphenol A from biomonitoring data. Within the predictability of the forward and reverse dosimetry estimations, the calculated daily intake was found to be far below established health benchmark levels (i.e., the suggested daily reported reference dose) with a wide (4 orders of magnitude) safety margin. These results suggest that the simplified PBPK models can be successfully applied to forward and reverse dosimetry estimations of tissue and/or blood exposures of tetrabromobisphenol A in humans after oral doses.
中文翻译:

使用简化的基于生理学的药代动力学模型从人源化肝脏小鼠数据推断出的人体中四溴双酚 A 的代谢特征
四溴双酚 A 是一种溴化阻燃剂,在世界范围内越来越普遍,并存在潜在的健康风险。在人源化肝小鼠中口服给药剂量后,四溴双酚 A 的调整动物生物监测当量使用已知的物种异速生长比例因子按比例放大至人类,以建立简化的基于生理学的药代动力学 (PBPK) 模型。分别在大鼠、对照小鼠和人源化肝小鼠中,吸收的四溴双酚 A在体内被轻度、中度和广泛代谢为葡萄糖醛酸。电脑模拟使用大鼠 PBPK 模型生成的血浆浓度曲线生成的四溴双酚 A 及其葡萄糖醛酸苷的估计肝脏暴露量与报告值一致。通过检测口服高剂量四溴双酚 A (1000 mg/kg) 后小鼠血浆中的人白蛋白 mRNA 来评估四溴双酚 A 引起的人源化肝小鼠肝损伤的程度。使用两个人类 PBPK 模型(基于人源化肝小鼠模型并通过优化报告的人类四溴双酚 A 葡萄糖醛酸苷酸血浆浓度的输入参数)进行反向剂量分析,以估计基于报告的人类四溴双酚 A 每日摄入量来自生物监测数据的总四溴双酚 A 的血清浓度。在正向和反向剂量学估计的可预测性范围内,发现计算出的每日摄入量远低于既定的健康基准水平(即建议的每日报告参考剂量),安全范围很宽(4 个数量级)。这些结果表明,简化的 PBPK 模型可以成功地应用于人类口服四溴双酚 A 组织和/或血液暴露的正向和反向剂量学估计。
更新日期:2020-11-16
中文翻译:

使用简化的基于生理学的药代动力学模型从人源化肝脏小鼠数据推断出的人体中四溴双酚 A 的代谢特征
四溴双酚 A 是一种溴化阻燃剂,在世界范围内越来越普遍,并存在潜在的健康风险。在人源化肝小鼠中口服给药剂量后,四溴双酚 A 的调整动物生物监测当量使用已知的物种异速生长比例因子按比例放大至人类,以建立简化的基于生理学的药代动力学 (PBPK) 模型。分别在大鼠、对照小鼠和人源化肝小鼠中,吸收的四溴双酚 A在体内被轻度、中度和广泛代谢为葡萄糖醛酸。电脑模拟使用大鼠 PBPK 模型生成的血浆浓度曲线生成的四溴双酚 A 及其葡萄糖醛酸苷的估计肝脏暴露量与报告值一致。通过检测口服高剂量四溴双酚 A (1000 mg/kg) 后小鼠血浆中的人白蛋白 mRNA 来评估四溴双酚 A 引起的人源化肝小鼠肝损伤的程度。使用两个人类 PBPK 模型(基于人源化肝小鼠模型并通过优化报告的人类四溴双酚 A 葡萄糖醛酸苷酸血浆浓度的输入参数)进行反向剂量分析,以估计基于报告的人类四溴双酚 A 每日摄入量来自生物监测数据的总四溴双酚 A 的血清浓度。在正向和反向剂量学估计的可预测性范围内,发现计算出的每日摄入量远低于既定的健康基准水平(即建议的每日报告参考剂量),安全范围很宽(4 个数量级)。这些结果表明,简化的 PBPK 模型可以成功地应用于人类口服四溴双酚 A 组织和/或血液暴露的正向和反向剂量学估计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号