当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amide–Amine Replacement in Indole-2-carboxamides Yields Potent Mycobactericidal Agents with Improved Water Solubility
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-11-16 , DOI: 10.1021/acsmedchemlett.0c00588 Yu Jia Tan 1 , Ming Li 1 , Gregory Adrian Gunawan 1 , Samuel Agyei Nyantakyi 1 , Thomas Dick 2, 3 , Mei-Lin Go 1 , Yulin Lam 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-11-16 , DOI: 10.1021/acsmedchemlett.0c00588 Yu Jia Tan 1 , Ming Li 1 , Gregory Adrian Gunawan 1 , Samuel Agyei Nyantakyi 1 , Thomas Dick 2, 3 , Mei-Lin Go 1 , Yulin Lam 1
Affiliation
|
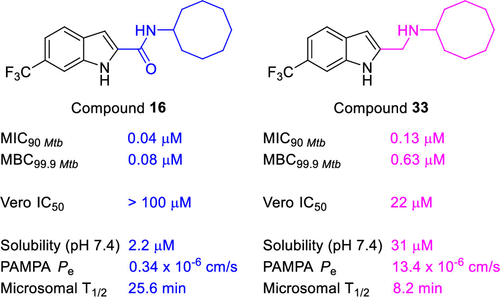
Indolecarboxamides are potent but poorly soluble mycobactericidal agents. Here we found that modifying the incipient scaffold by amide–amine substitution and replacing the indole ring with benzothiophene or benzoselenophene led to striking (10–20-fold) improvements in solubility. Potent activity could be achieved without the carboxamide linker but not in the absence of the indole ring. The indolylmethylamine, N-cyclooctyl-6-trifluoromethylindol-2-ylmethylamine (33, MIC90Mtb 0.13 μM, MBC99.9Mtb 0.63 μM), exemplifies a promising member that is more soluble and equipotent to its carboxamide equivalent. It is also an inhibitor of the mycolate transporter MmpL3, a property shared by the methylamines of benzothiophene and benzoselenophene.
中文翻译:

Indole-2-carboxamides 中的酰胺-胺替代可产生具有改善水溶性的强效分枝杆菌剂
吲哚羧酰胺是强效但难溶的分枝杆菌剂。在这里,我们发现通过酰胺-胺取代修饰初始支架并用苯并噻吩或苯并硒吩取代吲哚环导致溶解度显着提高(10-20 倍)。在没有羧酰胺接头的情况下可以实现有效的活性,但在没有吲哚环的情况下则无法实现。吲哚甲胺,N-环辛基-6-三氟甲基吲哚-2-基甲胺(33,MIC 90 Mtb 0.13 μM,MBC 99.9 Mtb0.63 μM),举例说明了一个有前途的成员,它比其羧酰胺等效物更易溶解和等效。它也是分枝菌酸盐转运蛋白 MmpL3 的抑制剂,这是苯并噻吩和苯并硒吩的甲胺共有的特性。
更新日期:2020-11-16
中文翻译:

Indole-2-carboxamides 中的酰胺-胺替代可产生具有改善水溶性的强效分枝杆菌剂
吲哚羧酰胺是强效但难溶的分枝杆菌剂。在这里,我们发现通过酰胺-胺取代修饰初始支架并用苯并噻吩或苯并硒吩取代吲哚环导致溶解度显着提高(10-20 倍)。在没有羧酰胺接头的情况下可以实现有效的活性,但在没有吲哚环的情况下则无法实现。吲哚甲胺,N-环辛基-6-三氟甲基吲哚-2-基甲胺(33,MIC 90 Mtb 0.13 μM,MBC 99.9 Mtb0.63 μM),举例说明了一个有前途的成员,它比其羧酰胺等效物更易溶解和等效。它也是分枝菌酸盐转运蛋白 MmpL3 的抑制剂,这是苯并噻吩和苯并硒吩的甲胺共有的特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号