当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent developments in catalytic amide bond formation
Peptide Science ( IF 1.5 ) Pub Date : 2020-11-16 , DOI: 10.1002/pep2.24210 Mihajlo Todorovic 1 , David M. Perrin 1
Peptide Science ( IF 1.5 ) Pub Date : 2020-11-16 , DOI: 10.1002/pep2.24210 Mihajlo Todorovic 1 , David M. Perrin 1
Affiliation
|
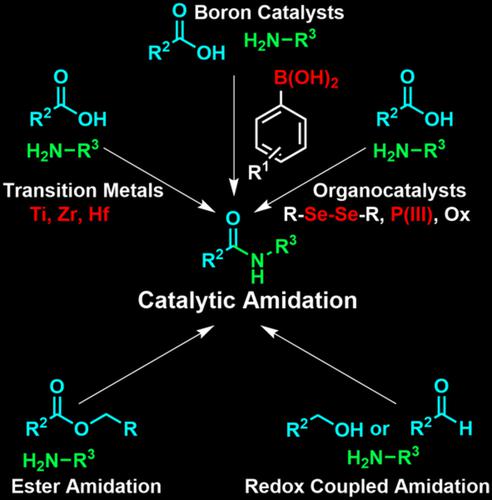
Amide bond forming reactions are critical for both polypeptide synthesis and medicinal chemistry. Most current approaches for amidation employ stoichiometric activating agents, but such methods are neither atom economical nor synthetically elegant. Catalytic approaches for amidation are potentially green and more ideal substitutes for current standard methods and thus are the subject of this review. Such methods face significant thermodynamic and kinetic barriers and have, as a result, historically conceded the use of elevated temperatures and dehydrating agents or lacked broad and relevant substrate scopes from the perspective of peptide chemistry. Recent advancements in methods for both direct amidation (the coupling of a carboxylic acid and an amine) and indirect amidation (the coupling of other partners resulting in an amide bond) based on aryl boronic acids, transition metals and organocatalysis for the former and ester amidation and redox‐coupled amidation for the later, address these previous shortcomings and are examined therein.
中文翻译:

催化酰胺键形成的最新进展
酰胺键形成反应对于多肽合成和药物化学都至关重要。当前大多数酰胺化方法使用化学计量的活化剂,但是这种方法既不经济又不经济。酰胺化的催化方法可能是绿色的,并且是当前标准方法的更理想替代品,因此是本综述的主题。这样的方法面临显着的热力学和动力学障碍,结果,从历史上讲,从肽化学的角度来看,它们已禁止使用高温和脱水剂,或者缺乏广泛而相关的底物范围。
更新日期:2020-12-01
中文翻译:

催化酰胺键形成的最新进展
酰胺键形成反应对于多肽合成和药物化学都至关重要。当前大多数酰胺化方法使用化学计量的活化剂,但是这种方法既不经济又不经济。酰胺化的催化方法可能是绿色的,并且是当前标准方法的更理想替代品,因此是本综述的主题。这样的方法面临显着的热力学和动力学障碍,结果,从历史上讲,从肽化学的角度来看,它们已禁止使用高温和脱水剂,或者缺乏广泛而相关的底物范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号