当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacokinetics of salmeterol and its main metabolite α‐hydroxysalmeterol after acute and chronic dry powder inhalation in exercising endurance‐trained men: Implications for doping control
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-11-17 , DOI: 10.1002/dta.2978 Søren Jessen 1 , Victoria Becker 2 , Sebastian Rzeppa 3 , Vibeke Backer 4 , Kasper Høtoft Bengtsen 1 , Ingunn Hullstein 3 , Yvette Dehnes 3 , Morten Hostrup 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2020-11-17 , DOI: 10.1002/dta.2978 Søren Jessen 1 , Victoria Becker 2 , Sebastian Rzeppa 3 , Vibeke Backer 4 , Kasper Høtoft Bengtsen 1 , Ingunn Hullstein 3 , Yvette Dehnes 3 , Morten Hostrup 1
Affiliation
|
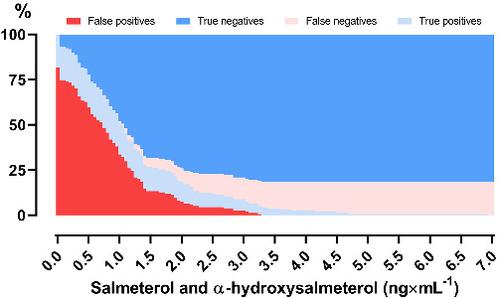
As of 2020, use of beta2‐agonist salmeterol is restricted by the World Anti‐Doping Agency (WADA) and is only permitted by inhalation at therapeutic doses not exceeding 200 μg in 24 h. In contrast to beta2‐agonists salbutamol and formoterol, WADA has not established a urine threshold for salmeterol despite its muscle hypertrophic actions observed in animals. Herein, we investigated plasma (0–4 h) and urine (0–24 h) concentrations (by ultra‐high‐performance liquid chromatography–tandem mass spectrometry [UHPLC–MS/MS]) of salmeterol and α‐hydroxysalmeterol after dry powder inhalation at supratherapeutic (400 μg) and high therapeutic (200 μg) doses, and after seven consecutive days of therapeutic inhalation (200 μg × day−1) in 11 healthy endurance‐trained men. During each trial, participants inhaled salmeterol before 1½ h moderate‐intensity cycling. Mean ± SD maximum urine concentrations of salmeterol unadjusted for specific gravity reached 4.0 ± 1.6, 2.1 ± 1.5, and 2.2 ± 1.1 ng × ml−1 for 400 μg, 200 μg, and seven consecutive days of 200 μg, respectively, with corresponding maximum urine concentrations of α‐hydroxysalmeterol being 11.6 ± 6.1, 5.7 ± 4.6, and 6.5 ± 2.6 ng × ml−1. Within the relevant window for doping control (first 6 h post‐inhalation), the present data (119 samples), along with 64 biobank urine samples, showed that a combined salmeterol and α‐hydroxysalmeterol urine threshold with equal cut‐offs of 3.3 ng × ml−1 was superior to a salmeterol‐only threshold to discriminate therapeutic (200 μg) from supratherapeutic use (400 μg) with a sensitivity of 24% with 0% false positives when applying the WADA technical document (TD2019DL.v2) method of specific gravity adjustment. Thus, a combination of urine salmeterol and α‐hydroxysalmeterol concentrations may be suitable for discriminating between therapeutic and supratherapeutic prohibited inhalation of salmeterol.
中文翻译:

沙美特罗及其主要代谢物 α-羟基沙美特罗在耐力训练男性急性和慢性吸入干粉后的药代动力学:对兴奋剂控制的影响
如2020年,使用β的2 -激动剂的沙美特罗是由世界反兴奋剂机构(WADA)限制,并且通过吸入在治疗剂量时,才允许在24小时不超过200微克。与 β 2受体激动剂沙丁胺醇和福莫特罗相比,尽管在动物身上观察到了沙美特罗的肌肉肥大作用,但 WADA 尚未确定沙美特罗的尿阈值。在此,我们研究了干粉后沙美特罗和 α-羟基沙美特罗的血浆(0-4 小时)和尿液(0-24 小时)浓度(通过超高效液相色谱-串联质谱 [UHPLC-MS/MS])以超治疗剂量(400 μg)和高治疗剂量(200 μg)吸入,并在连续 7 天的治疗性吸入(200 μg × 天-1) 在 11 名经过耐力训练的健康男性中。在每次试验中,参与者在 1.5 小时中等强度骑行前吸入沙美特罗。未经比重调整的沙美特罗平均 ± SD 最大尿液浓度分别达到 4.0 ± 1.6、2.1 ± 1.5 和 2.2 ± 1.1 ng × ml -1 400 μg、200 μg 和连续 7 天的 200 μg,相应的最大值α-羟基沙美特罗的尿液浓度为 11.6 ± 6.1、5.7 ± 4.6 和 6.5 ± 2.6 ng × ml -1。在兴奋剂控制的相关窗口内(吸入后前 6 小时),目前的数据(119 份样本)以及 64 份生物样本库尿液样本显示沙美特罗和 α-羟基沙美特罗的联合尿液阈值具有相同的截止值 3.3 ng × 毫升-1当应用 WADA 技术文件 (TD2019DL.v2) 比重调整方法时,在区分治疗剂(200 μg)和超治疗剂使用(400 μg)方面优于沙美特罗的阈值,灵敏度为 24%,假阳性率为 0%。因此,尿液沙美特罗和 α-羟基沙美特罗浓度的组合可能适用于区分治疗性和超治疗性吸入沙美特罗。
更新日期:2020-11-17
中文翻译:

沙美特罗及其主要代谢物 α-羟基沙美特罗在耐力训练男性急性和慢性吸入干粉后的药代动力学:对兴奋剂控制的影响
如2020年,使用β的2 -激动剂的沙美特罗是由世界反兴奋剂机构(WADA)限制,并且通过吸入在治疗剂量时,才允许在24小时不超过200微克。与 β 2受体激动剂沙丁胺醇和福莫特罗相比,尽管在动物身上观察到了沙美特罗的肌肉肥大作用,但 WADA 尚未确定沙美特罗的尿阈值。在此,我们研究了干粉后沙美特罗和 α-羟基沙美特罗的血浆(0-4 小时)和尿液(0-24 小时)浓度(通过超高效液相色谱-串联质谱 [UHPLC-MS/MS])以超治疗剂量(400 μg)和高治疗剂量(200 μg)吸入,并在连续 7 天的治疗性吸入(200 μg × 天-1) 在 11 名经过耐力训练的健康男性中。在每次试验中,参与者在 1.5 小时中等强度骑行前吸入沙美特罗。未经比重调整的沙美特罗平均 ± SD 最大尿液浓度分别达到 4.0 ± 1.6、2.1 ± 1.5 和 2.2 ± 1.1 ng × ml -1 400 μg、200 μg 和连续 7 天的 200 μg,相应的最大值α-羟基沙美特罗的尿液浓度为 11.6 ± 6.1、5.7 ± 4.6 和 6.5 ± 2.6 ng × ml -1。在兴奋剂控制的相关窗口内(吸入后前 6 小时),目前的数据(119 份样本)以及 64 份生物样本库尿液样本显示沙美特罗和 α-羟基沙美特罗的联合尿液阈值具有相同的截止值 3.3 ng × 毫升-1当应用 WADA 技术文件 (TD2019DL.v2) 比重调整方法时,在区分治疗剂(200 μg)和超治疗剂使用(400 μg)方面优于沙美特罗的阈值,灵敏度为 24%,假阳性率为 0%。因此,尿液沙美特罗和 α-羟基沙美特罗浓度的组合可能适用于区分治疗性和超治疗性吸入沙美特罗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号