当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Byproducts formed During Thiol‐Acrylate Reactions Promoted by Nucleophilic Aprotic Amines: Persistent or Reactive?
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-11-17 , DOI: 10.1002/cplu.202000590 Vasileios Drogkaris 1 , Brian H. Northrop 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-11-17 , DOI: 10.1002/cplu.202000590 Vasileios Drogkaris 1 , Brian H. Northrop 1
Affiliation
|
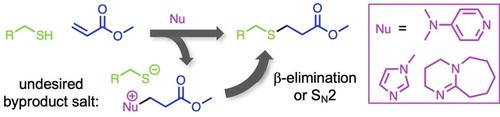
The nucleophile‐initiated mechanism of thiol‐Michael reactions naturally leads to the formation of undesired nucleophile byproducts. Three aza‐Michael compounds representing nucleophile byproducts of thiol‐acrylate reactions initiated by 4‐dimethylaminopyridine (DMAP), 1‐methylimidazole (MIM), and 1,8‐diazabicyclo[5.4.0]undec‐7‐ene (DBU) have been synthesized and their reactivity in the presence of thiolate has been investigated. Spectroscopic analysis shows that each nucleophile byproduct reacts with thiolate to produce a desired thiol‐acrylate product along with liberated aprotic amines DMAP, MIM, or DBU, thus demonstrating that these byproducts are reactive rather than persistent. Density functional theoretical computations support experimental observations and predict that a β‐elimination mechanism is favored for converting each nucleophile byproduct into a desired thiol‐acrylate product, though an SN2 process can be competitive (i. e. within <2.5 kcal/mol) in less polar solvents.
中文翻译:

亲核性非质子胺促进的丙烯酸-丙烯酸酯反应过程中形成的副产物:持久性还是反应性?
亲硫基-迈克尔反应的亲核引发机制自然会导致形成不希望的亲核副产物。三种由氮杂迈克尔基化合物代表的巯基丙烯酸酯反应的亲核副产物是由4-二甲基氨基吡啶(DMAP),1-甲基咪唑(MIM)和1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)引发的已经研究了它们的合成及其在硫醇盐存在下的反应性。光谱分析表明,每种亲核试剂副产物与硫醇盐反应生成所需的巯基丙烯酸酯产物以及释放的非质子胺DMAP,MIM或DBU,从而证明这些副产物是反应性的而不是持久的。N 2工艺在极性较小的溶剂中可能具有竞争力(即<2.5 kcal / mol以内)。
更新日期:2020-11-17
中文翻译:

亲核性非质子胺促进的丙烯酸-丙烯酸酯反应过程中形成的副产物:持久性还是反应性?
亲硫基-迈克尔反应的亲核引发机制自然会导致形成不希望的亲核副产物。三种由氮杂迈克尔基化合物代表的巯基丙烯酸酯反应的亲核副产物是由4-二甲基氨基吡啶(DMAP),1-甲基咪唑(MIM)和1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)引发的已经研究了它们的合成及其在硫醇盐存在下的反应性。光谱分析表明,每种亲核试剂副产物与硫醇盐反应生成所需的巯基丙烯酸酯产物以及释放的非质子胺DMAP,MIM或DBU,从而证明这些副产物是反应性的而不是持久的。N 2工艺在极性较小的溶剂中可能具有竞争力(即<2.5 kcal / mol以内)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号