当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Appended Aromatic Moieties in Flexible Bis‐3‐chloropiperidines Confer Tropism against Pancreatic Cancer Cells
ChemMedChem ( IF 3.6 ) Pub Date : 2020-11-17 , DOI: 10.1002/cmdc.202000814 Caterina Carraro 1 , Tim Helbing 2 , Alexander Francke 2 , Ivonne Zuravka 2 , Alice Sosic 1 , Michele De Franco 1 , Valentina Gandin 1 , Barbara Gatto 1 , D Richard Göttlich 2
ChemMedChem ( IF 3.6 ) Pub Date : 2020-11-17 , DOI: 10.1002/cmdc.202000814 Caterina Carraro 1 , Tim Helbing 2 , Alexander Francke 2 , Ivonne Zuravka 2 , Alice Sosic 1 , Michele De Franco 1 , Valentina Gandin 1 , Barbara Gatto 1 , D Richard Göttlich 2
Affiliation
|
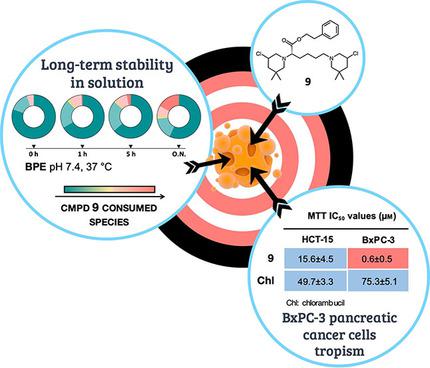
Nitrogen mustards (NMs) are an old but still largely diffused class of anticancer drugs. However, spreading mechanisms of resistance undermine their efficacy and therapeutic applicability. To expand their antitumour value, we developed bis‐3‐chloropiperidines (B‐CePs), a new class of mustard‐based alkylating agent, and we recently reported the striking selectivity for BxPC‐3 pancreatic tumour cells of B‐CePs bearing aromatic moieties embedded in the linker. In this study, we demonstrate that such tropism is shared by bis‐3‐chloropiperidines bearing appended aromatic groups in flexible linkers, whereas esters substituted by aliphatic groups or by efficient DNA‐interacting groups are potent but nonselective cytotoxic agents. Besides, we describe how the critical balance between water stability and DNA reactivity can affect the properties of bis‐3‐chloropiperidines. Together, these findings support the exploitation of B‐CePs as potential antitumour clinical candidates.
中文翻译:

柔性双-3-氯哌啶中附加的芳香族部分赋予抗胰腺癌细胞的趋向性
氮芥(NM)是一类古老但仍然广泛传播的抗癌药物。然而,耐药性的传播机制破坏了它们的功效和治疗适用性。为了扩大其抗肿瘤价值,我们开发了双-3-氯哌啶 (B-CePs),一种新型芥子基烷化剂,并且我们最近报道了带有芳香族部分的 B-CePs 对 BxPC-3 胰腺肿瘤细胞的惊人选择性嵌入链接器中。在这项研究中,我们证明了这种向性是在柔性连接体中带有附加芳香族基团的双-3-氯哌啶所共有的,而被脂肪族基团或有效的DNA相互作用基团取代的酯是有效但非选择性的细胞毒性剂。此外,我们还描述了水稳定性和 DNA 反应性之间的关键平衡如何影响双-3-氯哌啶的性质。总之,这些发现支持 B-CeP 作为潜在的抗肿瘤临床候选药物的开发。
更新日期:2020-11-17
中文翻译:

柔性双-3-氯哌啶中附加的芳香族部分赋予抗胰腺癌细胞的趋向性
氮芥(NM)是一类古老但仍然广泛传播的抗癌药物。然而,耐药性的传播机制破坏了它们的功效和治疗适用性。为了扩大其抗肿瘤价值,我们开发了双-3-氯哌啶 (B-CePs),一种新型芥子基烷化剂,并且我们最近报道了带有芳香族部分的 B-CePs 对 BxPC-3 胰腺肿瘤细胞的惊人选择性嵌入链接器中。在这项研究中,我们证明了这种向性是在柔性连接体中带有附加芳香族基团的双-3-氯哌啶所共有的,而被脂肪族基团或有效的DNA相互作用基团取代的酯是有效但非选择性的细胞毒性剂。此外,我们还描述了水稳定性和 DNA 反应性之间的关键平衡如何影响双-3-氯哌啶的性质。总之,这些发现支持 B-CeP 作为潜在的抗肿瘤临床候选药物的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号