当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility enhancement, solvent effect and thermodynamic analysis of pazopanib in co-solvent mixtures
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.jct.2020.106343 Hongwei Shi , Yong Xie , Jigui Xu , Jun Zhu , Cong Wang , Hongyan Wang
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.jct.2020.106343 Hongwei Shi , Yong Xie , Jigui Xu , Jun Zhu , Cong Wang , Hongyan Wang
|
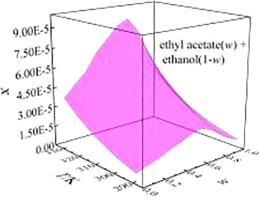
Abstract As is known to all, co-solvency or solvent mixing is the most common and easy-to-use method in the pharmaceutical industry for the improvement of solubility of poorly soluble drugs. The aim of this investigation was to determine the solubility data of pazopanib in (ethyl acetate + ethanol) and (ethyl acetate + 2-propanol) binary mixture solvents at various temperatures ranging from 288.15 K to 328.15 K, and correlated by Jouyban-acree model and Apelblat-Jouyban-Acree model. The results showed that solubility of pazopanib increased with the increasing temperature, and the co-solvency phenomenon was occurred in the dissolution process of pazopanib in two mixed solvents. The maximum solubility value appeared at w = 0.60 (the mass fraction of ethyl acetate in mixed solvents systems). At 288.15 K, solubility was increased by 4.37 and 3.94 times. The correlation results show that the maximum values of RAD and RMSD are no more than 5.13% and 3.37×10-6, respectively. The thermodynamic models can all correlate the solubility behaviour of pazopanib in pure and mixed solvents mixtures well. In addition, from the solvent effect results, nonspecific dipolarity/polarizability interactions are favorable to dissolution, and the nonspecific dipolarity/polarizability interactions had a great contribution to dissolution. Moreover, apparent thermodynamic quantities of pazopanib dissolution process in the ethyl acetate + ethanol/2-propanol mixtures, including entropy, enthalpy and Gibbs energy were computed using van't Hoff and Gibbs equations. The results of apparent dissolution properties are all positive which indicate the dissolution of pazopanib in two mixed solvents is an endothermic and entropy-increasing process and the enthalpy is the main contributor to standard Gibbs energy of solution process in all compositions. The discovery of co-solvency phenomenon is important to optimize the purification and improve bioavailability of pazopanib, and the solvent mixing is a common, easy-to-use and effective method to improve the solubility of pazopanib.
中文翻译:

帕唑帕尼在共溶剂混合物中的溶解度增强、溶剂效应和热力学分析
摘要 众所周知,共溶或溶剂混合是制药工业中提高难溶性药物溶解度最常用、最简便的方法。本研究的目的是确定帕唑帕尼在(乙酸乙酯 + 乙醇)和(乙酸乙酯 + 2-丙醇)二元混合溶剂中在 288.15 K 至 328.15 K 的不同温度下的溶解度数据,并通过 Jouyban-acree 模型进行关联和 Apelblat-Jouyban-Acree 模型。结果表明,帕唑帕尼的溶解度随着温度的升高而增加,帕唑帕尼在两种混合溶剂中的溶解过程中出现了共溶现象。最大溶解度值出现在 w = 0.60(乙酸乙酯在混合溶剂体系中的质量分数)。在 288.15 K 时,溶解度增加了 4.37 和 3。94次。相关结果表明,RAD和RMSD的最大值分别不超过5.13%和3.37×10-6。热力学模型都可以很好地关联帕唑帕尼在纯溶剂和混合溶剂混合物中的溶解度行为。此外,从溶剂效应结果来看,非特异性偶极/极化相互作用有利于溶解,非特异性偶极/极化相互作用对溶解有很大贡献。此外,帕唑帕尼在乙酸乙酯 + 乙醇/2-丙醇混合物中的溶解过程的表观热力学量,包括熵、焓和吉布斯能是使用范特霍夫和吉布斯方程计算的。表观溶出特性的结果都是正的,这表明帕唑帕尼在两种混合溶剂中的溶出是一个吸热和熵增加的过程,并且焓是所有组合物中标准吉布斯溶解过程的主要贡献者。共溶现象的发现对于优化帕唑帕尼的纯化和提高生物利用度具有重要意义,而溶剂混合是提高帕唑帕尼溶解度的常用、易用且有效的方法。
更新日期:2021-04-01
中文翻译:

帕唑帕尼在共溶剂混合物中的溶解度增强、溶剂效应和热力学分析
摘要 众所周知,共溶或溶剂混合是制药工业中提高难溶性药物溶解度最常用、最简便的方法。本研究的目的是确定帕唑帕尼在(乙酸乙酯 + 乙醇)和(乙酸乙酯 + 2-丙醇)二元混合溶剂中在 288.15 K 至 328.15 K 的不同温度下的溶解度数据,并通过 Jouyban-acree 模型进行关联和 Apelblat-Jouyban-Acree 模型。结果表明,帕唑帕尼的溶解度随着温度的升高而增加,帕唑帕尼在两种混合溶剂中的溶解过程中出现了共溶现象。最大溶解度值出现在 w = 0.60(乙酸乙酯在混合溶剂体系中的质量分数)。在 288.15 K 时,溶解度增加了 4.37 和 3。94次。相关结果表明,RAD和RMSD的最大值分别不超过5.13%和3.37×10-6。热力学模型都可以很好地关联帕唑帕尼在纯溶剂和混合溶剂混合物中的溶解度行为。此外,从溶剂效应结果来看,非特异性偶极/极化相互作用有利于溶解,非特异性偶极/极化相互作用对溶解有很大贡献。此外,帕唑帕尼在乙酸乙酯 + 乙醇/2-丙醇混合物中的溶解过程的表观热力学量,包括熵、焓和吉布斯能是使用范特霍夫和吉布斯方程计算的。表观溶出特性的结果都是正的,这表明帕唑帕尼在两种混合溶剂中的溶出是一个吸热和熵增加的过程,并且焓是所有组合物中标准吉布斯溶解过程的主要贡献者。共溶现象的发现对于优化帕唑帕尼的纯化和提高生物利用度具有重要意义,而溶剂混合是提高帕唑帕尼溶解度的常用、易用且有效的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号