Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.tet.2020.131743 Paul R. Mears , Eric J. Thomas
|
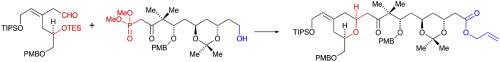
The stereoselective synthesis of a carboxylic acid ester corresponding to the C1–C16 fragment of bryostatin, with 4-methoxybenzyl (PMB) protection for the 7-hydroxyl group, is reported. The key steps included a Horner-Wadsworth-Emmons reaction between (5R)-3-[(E)-2-tri-isopropylsilyloxyethylidene]-6-(4-methoxybenzyloxy)-5-triethylsilyloxyhexanal and dimethyl (4S,6R,8S)-10-hydroxy-6,8-di-O-isopropylidene-4-(4-methoxybenzyloxy)-3,3-dimethyl-2-oxodecan-1-yl phosphonate, that gave the corresponding (E)-alkene, followed by selective cleavage of the triethylsilyl ether and cyclisation to give the required 2,6-cis-disubstituted 4-[(Z)-tri-isopropylsilyloxyethylide]tetrahydropyran. Oxidation of the primary alcohol gave the corresponding carboxylic acid that was converted into the required allyl ester.
中文翻译:

bryostatin C1 – C16片段的合成,以掺入20,20氟化类似物
据报道,立体选择性合成了与抑霉菌素C1-C16片段相对应的羧酸酯,其中4-羟基甲氧基苄基(PMB)保护7-羟基。关键步骤包括(5 R)-3-[(E)-2-三异丙基甲硅烷氧基乙叉基] -6-(4-甲氧基苄氧基)-5-三乙基甲硅烷氧基己醛与二甲基(4 S,6 R之间的Horner-Wadsworth-Emmons反应,8 S)-10-羟基-6,8-二-O-异亚丙基-4-(4-甲氧基苄氧基)-3,3-二甲基-2-氧杂癸-1-基膦酸酯,得到相应的(E)-烯烃,然后选择性裂解三乙基甲硅烷基醚并环化,得到所需的2,6-顺式-二取代的4-[(Z)-三异丙基甲硅烷氧基乙氧基]四氢吡喃。伯醇的氧化得到相应的羧酸,将其转化为所需的烯丙基酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号