Tetrahedron ( IF 2.1 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.tet.2020.131772 Lucia Očenášová , Mariana Budovská , Peter Očenáš , Nataša Tomášková , Martina Bago Pilátová , Ján Mojžiš
|
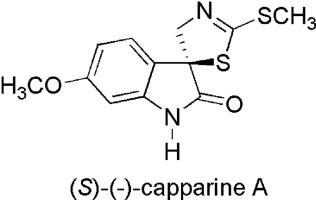
Substances containing a spirooxindole framework display important biological activities. Natural alkaloid capparine A [(S)-(−)-1] has an anti-inflammatory effect. In the present study, attention has been paid to the first total synthesis of natural capparine A [(S)-(−)-1]. Racemic capparine A [(±)-1] was synthesized by bromospirocyclization of 6-methoxy-1-Boc-brassinin with water, followed by oxidation of obtained spirobrassinol derivatives and removal of the Boc group. Synthesized racemic capparine A [(±)-1] was enantioresolved by derivatization with (1R,2S,5R)-menthyl chloroformate, chromatographic separation of diastereoisomers and the cleavage of the chiral auxiliary using sodium methoxide. Screening of anti-proliferative activity against human cancer cells revealed no anti-proliferative activity of the capparine A [(S)-(−)-1].
中文翻译:

天然生物碱卡帕汀A的首次合成
含有螺虫醇骨架的物质显示出重要的生物学活性。天然生物碱卡帕汀A [(S)-(-)- 1 ]具有抗炎作用。在本研究中,已经关注了天然卡帕汀A [(S)-(-)- 1 ]的第一个全合成。外消旋卡帕汀A [(±)-1 ]的合成方法是:用水将6-甲氧基-1-Boc-brassinin进行溴螺环化,然后氧化所获得的螺油菜素醇衍生物,并除去Boc基团。合成的外消旋卡帕汀A [(±)-1 ]通过(1 R,2 S,5 R)-氯甲酸薄荷酯,非对映异构体的色谱分离和使用甲醇钠的手性助剂的裂解 对人癌细胞的抗增殖活性的筛选显示,没有卡帕汀A [(S)-(-)- 1 ]的抗增殖活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号