当前位置:
X-MOL 学术
›
Mater. Today Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly active non-noble electrocatalyst from Co2P/Ni2P nanohybrids for pH-universal hydrogen evolution reaction
Materials Today Physics ( IF 10.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.mtphys.2020.100314 D.Y. Li , L.L. Liao , H.Q. Zhou , Y. Zhao , F.M. Cai , J.S. Zeng , F. Liu , H. Wu , D.S. Tang , F. Yu
Materials Today Physics ( IF 10.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.mtphys.2020.100314 D.Y. Li , L.L. Liao , H.Q. Zhou , Y. Zhao , F.M. Cai , J.S. Zeng , F. Liu , H. Wu , D.S. Tang , F. Yu
|
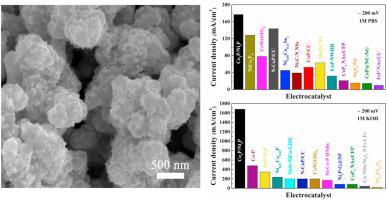
Abstract Many water sources including seawater, industrial wastewater and residential water are naturally promising ingredients for hydrogen production from water electrolysis, in which an efficient hydrogen-evolving electrocatalyst is required to work energetically under different pH environments. However, very few of non-noble electrocatalysts exhibit promising hydrogen-evolving activities in both neutral and alkaline solutions at present. Here we demonstrate that a highly porous hydrogen-evolving electrocatalyst, which is established by in-situ formation of Co2P/Ni2P nanohybrids with a nanometer size on a conductive CoNi foam, presents very outstanding pH-universal catalytic activities for hydrogen evolution in a wide pH range demanding extremely low overpotentials of 65.7 and 51 mV to yield 10 mA/cm2 with exceptionally operational durability in 1M phosphate buffer solution (PBS, pH ≈ 6.5) and 1M KOH (pH = 14), respectively, and 46 mV to deliver 20 mA/cm 2 stably in 0.5M H 2 SO 4 (pH ≈ 0.3). More interestingly, it is worth mentioning that this catalyst can bear huge current densities up to 177, 1700 and 1000 mA/cm 2 once the overpotential is increased to 0.2 V in neutral, alkaline and acidic solutions, respectively. These catalytic activities outperform most of the documented non-noble electrocatalysts composed of transition metal phosphides, selenides, sulfides, etc., and match or even surpass noble Pt catalysts. It probably represents the best hydrogen-evolving activity among the ever-reported Earth-abundant catalysts for HER hitherto, which is probably arisen from the large surface area, the exposure of numerous active sites and strong interfacial interactions between Co2P and Ni2P particles. This discovery may pave a new avenue toward the development of robust inexpensive electrocatalysts for hydrogen production in unfavorable neutral or alkaline media.
中文翻译:

来自 Co2P/Ni2P 纳米杂化物的高活性非贵金属电催化剂,用于 pH 通用析氢反应
摘要 海水、工业废水和生活用水等许多水源是水电解制氢的天然有前途的成分,其中需要高效的析氢电催化剂在不同的 pH 值环境下高效工作。然而,目前很少有非贵重的电催化剂在中性和碱性溶液中表现出有希望的析氢活性。在这里,我们证明了一种高度多孔的析氢电催化剂,它是通过在导电 CoNi 泡沫上原位形成纳米尺寸的 Co2P/Ni2P 纳米杂化物而建立的,在宽 pH 值条件下对析氢具有非常出色的 pH 通用催化活性范围要求 65 的极低过电位。7 和 51 mV 分别在 1M 磷酸盐缓冲溶液(PBS,pH ≈ 6.5)和 1M KOH(pH = 14)中产生 10 mA/cm2,具有出色的操作耐久性,46 mV 可在 0.5 中稳定提供 20 mA/cm 2 MH 2 SO 4 (pH ≈ 0.3)。更有趣的是,值得一提的是,一旦过电位在中性、碱性和酸性溶液中分别增加到 0.2 V,该催化剂可以承受高达 177、1700 和 1000 mA/cm 2 的巨大电流密度。这些催化活性优于大多数已记录的由过渡金属磷化物、硒化物、硫化物等组成的非贵金属电催化剂,并与贵金属铂催化剂相媲美甚至超过。它可能代表迄今为止报道的地球上丰富的 HER 催化剂中最好的析氢活性,这可能源于大表面积,许多活性位点的暴露以及 Co2P 和 Ni2P 颗粒之间的强界面相互作用。这一发现可能为开发用于在不利的中性或碱性介质中制氢的稳定且廉价的电催化剂开辟了一条新途径。
更新日期:2021-01-01
中文翻译:

来自 Co2P/Ni2P 纳米杂化物的高活性非贵金属电催化剂,用于 pH 通用析氢反应
摘要 海水、工业废水和生活用水等许多水源是水电解制氢的天然有前途的成分,其中需要高效的析氢电催化剂在不同的 pH 值环境下高效工作。然而,目前很少有非贵重的电催化剂在中性和碱性溶液中表现出有希望的析氢活性。在这里,我们证明了一种高度多孔的析氢电催化剂,它是通过在导电 CoNi 泡沫上原位形成纳米尺寸的 Co2P/Ni2P 纳米杂化物而建立的,在宽 pH 值条件下对析氢具有非常出色的 pH 通用催化活性范围要求 65 的极低过电位。7 和 51 mV 分别在 1M 磷酸盐缓冲溶液(PBS,pH ≈ 6.5)和 1M KOH(pH = 14)中产生 10 mA/cm2,具有出色的操作耐久性,46 mV 可在 0.5 中稳定提供 20 mA/cm 2 MH 2 SO 4 (pH ≈ 0.3)。更有趣的是,值得一提的是,一旦过电位在中性、碱性和酸性溶液中分别增加到 0.2 V,该催化剂可以承受高达 177、1700 和 1000 mA/cm 2 的巨大电流密度。这些催化活性优于大多数已记录的由过渡金属磷化物、硒化物、硫化物等组成的非贵金属电催化剂,并与贵金属铂催化剂相媲美甚至超过。它可能代表迄今为止报道的地球上丰富的 HER 催化剂中最好的析氢活性,这可能源于大表面积,许多活性位点的暴露以及 Co2P 和 Ni2P 颗粒之间的强界面相互作用。这一发现可能为开发用于在不利的中性或碱性介质中制氢的稳定且廉价的电催化剂开辟了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号