Biochimie ( IF 3.3 ) Pub Date : 2020-11-17 , DOI: 10.1016/j.biochi.2020.11.012 Cristina Algieri , Salvatore Nesci , Fabiana Trombetti , Micaela Fabbri , Vittoria Ventrella , Alessandra Pagliarani
|
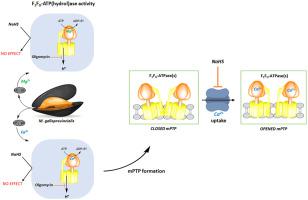
The molecular mechanisms which rule the formation and opening of the mitochondrial permeability transition pore (mPTP), the lethal mechanism which permeabilizes mitochondria to water and solutes and drives the cell to death, are still unclear and particularly little investigated in invertebrates. Since Ca2+ increase in mitochondria is accompanied by mPTP opening and the participation of the mitochondrial F1FO-ATPase in the mPTP is increasingly sustained, the substitution of the natural cofactor Mg2+ by Ca2+ in the F1FO-ATPase activation has been involved in the mPTP mechanism. In mussel midgut gland mitochondria the similar kinetic properties of the Mg2+- or Ca2+-dependent F1FO-ATPase activities, namely the same affinity for ATP and bi-site activation kinetics by the ATP substrate, in spite of the higher enzyme activity and coupling efficiency of the Mg2+-dependent F1FO-ATPase, suggest that both enzyme activities are involved in the bioenergetic machinery. Other than being a mitochondrial poison and environmental contaminant, sulfide at low concentrations acts as gaseous mediator and can induce post-translational modifications of proteins. The sulfide donor NaHS, at micromolar concentrations, does not alter the two F1FO-ATPase activities, but desensitizes the mPTP to Ca2+ input. Unexpectedly, NaHS, under the conditions tested, points out a chemical refractoriness of both F1FO-ATPase activities and a failed relationship between the Ca2+-dependent F1FO-ATPase and the mPTP in mussels. The findings suggest that mPTP role and regulation may be different in different taxa and that the F1FO-ATPase insensitivity to NaHS may allow mussels to cope with environmental sulfide.
中文翻译:

鹅耳My中肠中线粒体F 1 F O -ATPase和对硫离子的通透性转变孔响应
决定线粒体通透性过渡孔(mPTP)形成和开放的分子机制,尚不清楚致死机制,该机制使线粒体通透到水和溶质中,并导致细胞死亡,但在无脊椎动物中研究很少。由于线粒体中Ca 2+的增加伴随着mPTP的开放,并且线粒体F 1 F O -ATPase在mPTP中的参与越来越多,因此天然辅因子Mg 2+被F 1 F O中的Ca 2+取代。-ATPase激活已参与了mPTP机制。在贻贝中肠线粒体中,Mg具有相似的动力学性质2+ -或Ca 2+依赖性F 1 F O -ATPase活性,尽管Mg 2 + -具有较高的酶活性和偶联效率,但对ATP的亲和力和ATP底物的双位激活动力学相同。依赖的F 1 F O -ATPase,表明这两种酶的活性都参与了生物能机制。除了是线粒体毒物和环境污染物以外,低浓度的硫化物还充当气态介质,并可以诱导蛋白质的翻译后修饰。硫化物供体NaHS在微摩尔浓度下不会改变两个F 1 F O-ATPase活性,但使mPTP对Ca 2+输入不敏感。出乎意料的是,在所测试的条件下,NaHS指出了F 1 F O -ATPase活性的化学耐性,以及贻贝中Ca 2+依赖性F 1 F O -ATPase与mPTP之间的失败关系。这些发现表明,mPTP的作用和调控在不同的分类单元中可能有所不同,并且F 1 F O -ATPase对NaHS的不敏感性可能使贻贝应对环境中的硫化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号