当前位置:
X-MOL 学术
›
Atmos. Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical insight into the oxidation mechanism of NO2 and SO2 on TiO2 surface: The role of H2O, NH3 and SO42-
Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.atmosenv.2020.118068 Zehua Wang , Chenxi Zhang , Guochun Lv , Xiaomin Sun
Atmospheric Environment ( IF 4.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.atmosenv.2020.118068 Zehua Wang , Chenxi Zhang , Guochun Lv , Xiaomin Sun
|
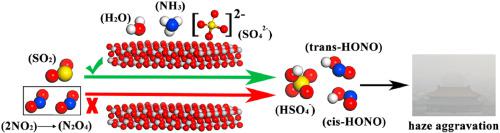
Abstract In this paper, density-functional theory (DFT) was employed to investigate the reaction mechanism of SO2 and NO2 and the important role of intermediate N2O4 formation. In addition, the effect of pure particulate surface (TiO2) and particulate surface containing other atmospheric components (H2O, NH3 and SO42−) on the conversion of SO2 and NO2 to sulfate also was analyzed. The detailed information that intermediate N2O4 acts as the oxidant was demonstrated in this oxidation reaction of SO2 and NO2 (as a rate-determining step). The pure particulate surface (TiO2) has hardly effect on the oxidation process of SO2 and N2O4. Whereas, different amounts of H2O and NH3 molecules as well as SO42−, contained on the particulate surface, can effectively reduce the activation energy of oxidation step. And, the optimal process is that SO2 is oxidized by cis-ONONO2 with the energy barrier of 4.54 kcal/mol when one NH3 molecule and one H2O molecule are contained on the TiO2(101) surface. When more H2O molecules are contained on particulate surface, SO2 tends to form HSO3− first. However, HSO3− is more difficult to be further oxidized by asy-ONONO2 than SO2. This study gains more insight into the contribution of SO2 and NO2 to haze and the potential impact of atmospheric constituents (including H2O/NH3/TiO2 and SO42−/TiO2) on the formation sulfate.
中文翻译:

NO2 和 SO2 在 TiO2 表面氧化机制的理论洞察:H2O、NH3 和 SO42-的作用
摘要 本文采用密度泛函理论(DFT)研究了SO2和NO2的反应机理以及中间体N2O4形成的重要作用。此外,还分析了纯颗粒表面(TiO2)和含有其他大气成分(H2O、NH3 和 SO42-)的颗粒表面对 SO2 和 NO2 转化为硫酸盐的影响。中间体 N2O4 作为氧化剂的详细信息在 SO2 和 NO2 的氧化反应(作为速率决定步骤)中得到证实。纯颗粒表面 (TiO2) 对 SO2 和 N2O4 的氧化过程几乎没有影响。而颗粒表面含有不同数量的 H2O 和 NH3 分子以及 SO42-,可以有效降低氧化步骤的活化能。和,最佳过程是当TiO2(101)表面含有1个NH3分子和1个H2O分子时,顺式ONONO2氧化SO2,能垒为4.54 kcal/mol。当颗粒表面含有更多的 H2O 分子时,SO2 往往首先形成 HSO3−。然而,与 SO2 相比,HSO3− 更难被 asy-ONONO2 进一步氧化。该研究更深入地了解 SO2 和 NO2 对雾霾的贡献以及大气成分(包括 H2O/NH3/TiO2 和 SO42-/TiO2)对形成硫酸盐的潜在影响。
更新日期:2021-02-01
中文翻译:

NO2 和 SO2 在 TiO2 表面氧化机制的理论洞察:H2O、NH3 和 SO42-的作用
摘要 本文采用密度泛函理论(DFT)研究了SO2和NO2的反应机理以及中间体N2O4形成的重要作用。此外,还分析了纯颗粒表面(TiO2)和含有其他大气成分(H2O、NH3 和 SO42-)的颗粒表面对 SO2 和 NO2 转化为硫酸盐的影响。中间体 N2O4 作为氧化剂的详细信息在 SO2 和 NO2 的氧化反应(作为速率决定步骤)中得到证实。纯颗粒表面 (TiO2) 对 SO2 和 N2O4 的氧化过程几乎没有影响。而颗粒表面含有不同数量的 H2O 和 NH3 分子以及 SO42-,可以有效降低氧化步骤的活化能。和,最佳过程是当TiO2(101)表面含有1个NH3分子和1个H2O分子时,顺式ONONO2氧化SO2,能垒为4.54 kcal/mol。当颗粒表面含有更多的 H2O 分子时,SO2 往往首先形成 HSO3−。然而,与 SO2 相比,HSO3− 更难被 asy-ONONO2 进一步氧化。该研究更深入地了解 SO2 和 NO2 对雾霾的贡献以及大气成分(包括 H2O/NH3/TiO2 和 SO42-/TiO2)对形成硫酸盐的潜在影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号