当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Modeling of Thioamide FRET Quenching by Including Conformational Restriction and Coulomb Coupling
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jpcb.0c06865 Jimin Yoon 1 , John J. Ferrie 1 , E. James Petersson 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jpcb.0c06865 Jimin Yoon 1 , John J. Ferrie 1 , E. James Petersson 1
Affiliation
|
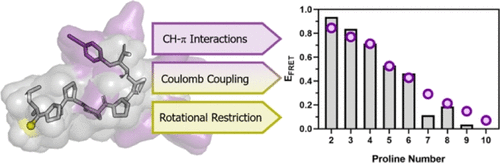
Thioamide-containing amino acids have been shown to quench a wide range of fluorophores through distinct mechanisms. Here, we quantitatively analyze the mechanism through which the thioamide functional group quenches the fluorescence of p-cyanophenylalanine (Cnf), tyrosine (Tyr), and tryptophan (Trp). By comparing PyRosetta simulations to published experiments performed on polyproline ruler peptides, we corroborate previous findings that both Cnf and Tyr quenching occurs via Förster resonance energy transfer (FRET), while Trp quenching occurs through an alternate mechanism such as Dexter transfer. Additionally, optimization of the peptide sampling scheme and comparison of thioamides attached to the peptide backbone and side chain revealed that the significant conformational restriction associated with the thioamide moiety results in a high sensitivity of the apparent FRET efficiency to underlying conformational differences. Moreover, by computing FRET efficiencies from structural models using a variety of approaches, we find that quantitative accuracy in the role of Coulomb coupling is required to explain contributions to the observed quenching efficiency from individual structures on a detailed level. Last, we demonstrate that these additional considerations improve our ability to predict thioamide quenching efficiencies observed during binding of thioamide-labeled peptides to fluorophore-labeled variants of calmodulin.
中文翻译:

包括构象限制和库仑偶联的改进硫代酰胺FRET淬火建模
含硫酰胺的氨基酸已经显示出通过不同的机理淬灭各种荧光团的方法。在这里,我们定量分析硫酰胺官能团淬灭对氰基苯丙氨酸(Cnf),酪氨酸(Tyr)和色氨酸(Trp)荧光的机理。通过将PyRosetta模拟与对多脯氨酸标尺肽进行的已发表实验进行比较,我们证实了先前的发现,即Cnf和Tyr淬灭均通过Förster共振能量转移(FRET),而Trp淬灭则通过诸如Dexter转移之类的替代机制发生。另外,肽采样方案的优化和与肽主链和侧链连接的硫酰胺的比较显示,与硫酰胺部分相关的显着构象限制导致表观FRET效率对潜在的构象差异高度敏感。此外,通过使用多种方法从结构模型计算FRET效率,我们发现需要使用库仑耦合作用的定量精度来详细解释各个结构对观察到的淬火效率的影响。持续,
更新日期:2020-11-25
中文翻译:

包括构象限制和库仑偶联的改进硫代酰胺FRET淬火建模
含硫酰胺的氨基酸已经显示出通过不同的机理淬灭各种荧光团的方法。在这里,我们定量分析硫酰胺官能团淬灭对氰基苯丙氨酸(Cnf),酪氨酸(Tyr)和色氨酸(Trp)荧光的机理。通过将PyRosetta模拟与对多脯氨酸标尺肽进行的已发表实验进行比较,我们证实了先前的发现,即Cnf和Tyr淬灭均通过Förster共振能量转移(FRET),而Trp淬灭则通过诸如Dexter转移之类的替代机制发生。另外,肽采样方案的优化和与肽主链和侧链连接的硫酰胺的比较显示,与硫酰胺部分相关的显着构象限制导致表观FRET效率对潜在的构象差异高度敏感。此外,通过使用多种方法从结构模型计算FRET效率,我们发现需要使用库仑耦合作用的定量精度来详细解释各个结构对观察到的淬火效率的影响。持续,











































 京公网安备 11010802027423号
京公网安备 11010802027423号