当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cooperative Effects Dominating the Thermodynamics and Kinetics of Surfactant Adsorption in Porous Media: From Lateral Interactions to Surface Aggregation
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jpcb.0c08226 Zaineb Zaafouri 1, 2 , Daniela Bauer 1 , Guillaume Batôt 1 , Carlos Nieto-Draghi 1 , Benoit Coasne 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-16 , DOI: 10.1021/acs.jpcb.0c08226 Zaineb Zaafouri 1, 2 , Daniela Bauer 1 , Guillaume Batôt 1 , Carlos Nieto-Draghi 1 , Benoit Coasne 2
Affiliation
|
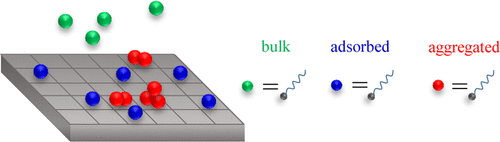
Surfactant adsorption in porous media remains poorly understood, as the microscopic collective behavior of these amphiphilic molecules leads to nonconventional phenomena with complex underlying kinetics/structural organization. Here, we develop a simple thermodynamic model, which captures this rich behavior by including cooperative effects to account for lateral interactions between adsorbed molecules and the formation of ordered or disordered self-assemblies. In more detail, this model relies on a kinetic approach, involving adsorption/desorption rates that depend on the surfactant surface concentration to account for facilitated or hindered adsorption at different adsorption stages. Using different surfactants/porous solids, adsorption on both strongly and weakly adsorbing surfaces is found to be accurately described with parameters that are readily estimated from available adsorption experiments. The validity of our physical approach is confirmed by showing that the inferred adsorption/desorption rates obey the quasi-chemical approximation for lateral adsorbate interactions. Such cooperative effects are shown to lead to adsorption kinetics that drastically depart from conventional frameworks (e.g., Henry, Langmuir, and Sips models).
中文翻译:

表面活性剂在多孔介质中吸附的热力学和动力学的协同效应:从横向相互作用到表面聚集
表面活性剂在多孔介质中的吸附仍然知之甚少,因为这些两亲分子的微观集体行为会导致具有复杂的基本动力学/结构组织的非常规现象。在这里,我们开发了一个简单的热力学模型,该模型通过包含协同效应来说明吸附分子之间的横向相互作用以及有序或无序自组装体的形成,从而捕获了这种丰富的行为。更详细地,该模型依赖于动力学方法,涉及取决于表面活性剂表面浓度的吸附/解吸速率,以说明在不同的吸附阶段促进或受阻的吸附。使用不同的表面活性剂/多孔固体,发现在强吸附和弱吸附表面上的吸附都可以通过可从现有吸附实验中轻松估算出的参数准确描述。我们的物理方法的有效性通过证明所推断的吸附/解吸速率符合侧向吸附物相互作用的准化学近似值得以证实。已显示出这种协同作用会导致吸附动力学大大偏离常规框架(例如,Henry,Langmuir和Sips模型)。
更新日期:2020-11-25
中文翻译:

表面活性剂在多孔介质中吸附的热力学和动力学的协同效应:从横向相互作用到表面聚集
表面活性剂在多孔介质中的吸附仍然知之甚少,因为这些两亲分子的微观集体行为会导致具有复杂的基本动力学/结构组织的非常规现象。在这里,我们开发了一个简单的热力学模型,该模型通过包含协同效应来说明吸附分子之间的横向相互作用以及有序或无序自组装体的形成,从而捕获了这种丰富的行为。更详细地,该模型依赖于动力学方法,涉及取决于表面活性剂表面浓度的吸附/解吸速率,以说明在不同的吸附阶段促进或受阻的吸附。使用不同的表面活性剂/多孔固体,发现在强吸附和弱吸附表面上的吸附都可以通过可从现有吸附实验中轻松估算出的参数准确描述。我们的物理方法的有效性通过证明所推断的吸附/解吸速率符合侧向吸附物相互作用的准化学近似值得以证实。已显示出这种协同作用会导致吸附动力学大大偏离常规框架(例如,Henry,Langmuir和Sips模型)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号