当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
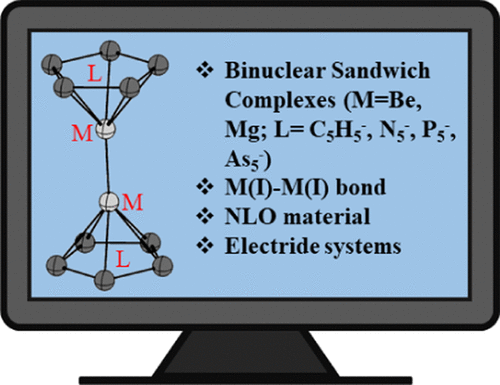
Electride Characteristics of Some Binuclear Sandwich Complexes of Alkaline Earth Metals, M2(η5-L)2 (M = Be, Mg; L = C5H5–, N5–, P5–, As5–)
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-11-15 , DOI: 10.1021/acs.jpca.0c08306 Prasenjit Das 1 , Pratim Kumar Chattaraj 1, 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-11-15 , DOI: 10.1021/acs.jpca.0c08306 Prasenjit Das 1 , Pratim Kumar Chattaraj 1, 2
Affiliation
|
Ab initio calculations have been performed for a series of binuclear sandwich complexes, M2(η5-L)2. It has been observed that the eclipsed and staggered conformations have almost equal amount of energies. The M–M bond lengths are comparable with those in the free M2 molecules (M = Be, Mg). The nuclear-independent chemical shift (NICS) values indicate the aromaticity of these complexes. The stability of Be2(η5-L)2 complexes is higher than that of the Mg2(η5-L)2 complexes. The natural bond orbital (NBO) analysis and electron density descriptors proved the existence of a single covalent M–M bond in an M22+ fragment. It has been observed that each M–M bond contains a non-nuclear attractor (NNA) at the center of the respective bond. The Laplacian of electron density [∇2ρ(r)] is negative at the NNAs. The energy decomposition analysis (EDA) showed that M22+ and 2L– represent the bonding interaction in the complexes. All of the designed binuclear sandwich complexes behave as electrides.
中文翻译:

一些双核夹心碱土金属的配合物,M的电子化合物特性2(η 5 -L)2(M =铍,镁; L = c ^ 5 ħ 5 -,N 5 -,P 5 - ,如5 - )
从头计算已经进行了一系列的双核夹心复合物的执行中,M 2(η 5 -L)2。已经观察到,黯淡和交错的构象具有几乎相等的能量。M–M键的长度与游离M 2分子中的键长度相当(M = Be,Mg)。不依赖核的化学位移(NICS)值表明这些配合物的芳香性。的是稳定性2(η 5 -L)2配合物是比镁更高的2(η 5 -L)2复合体。天然键轨道(NBO)分析和电子密度描述符证明了M 2 2+片段中存在单个共价M–M键。已经观察到,每个M–M键在相应键的中心都包含一个非核吸引子(NNA)。电子密度的拉普拉斯[∇ 2 ρ([R)]是在NNAs负。能量分解分析(EDA)表明,中号2 2+和2L -表示在复合物中的键合相互作用。所有设计的双核三明治复合物均表现为电子。
更新日期:2020-11-25
中文翻译:

一些双核夹心碱土金属的配合物,M的电子化合物特性2(η 5 -L)2(M =铍,镁; L = c ^ 5 ħ 5 -,N 5 -,P 5 - ,如5 - )
从头计算已经进行了一系列的双核夹心复合物的执行中,M 2(η 5 -L)2。已经观察到,黯淡和交错的构象具有几乎相等的能量。M–M键的长度与游离M 2分子中的键长度相当(M = Be,Mg)。不依赖核的化学位移(NICS)值表明这些配合物的芳香性。的是稳定性2(η 5 -L)2配合物是比镁更高的2(η 5 -L)2复合体。天然键轨道(NBO)分析和电子密度描述符证明了M 2 2+片段中存在单个共价M–M键。已经观察到,每个M–M键在相应键的中心都包含一个非核吸引子(NNA)。电子密度的拉普拉斯[∇ 2 ρ([R)]是在NNAs负。能量分解分析(EDA)表明,中号2 2+和2L -表示在复合物中的键合相互作用。所有设计的双核三明治复合物均表现为电子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号