当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autocatalyzed oxidation of d‐glucitol by alkaline copper (III) periodate complex: A kinetic and mechanistic approach
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-11-15 , DOI: 10.1002/poc.4146 Shreekant M. Patil 1 , Atmanand M. Bagoji 1 , Santosh B. Konnur 1 , Sharanappa T. Nandibewoor 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-11-15 , DOI: 10.1002/poc.4146 Shreekant M. Patil 1 , Atmanand M. Bagoji 1 , Santosh B. Konnur 1 , Sharanappa T. Nandibewoor 1
Affiliation
|
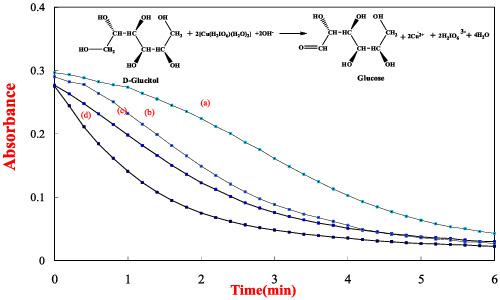
An autocatalyzed oxidation of d‐glucitol (DG) by diperiodatocuprate (III) (DPC) in aqueous alkaline medium at a constant ionic strength of 0.06 mol dm−3 was studied spectrophotometrically. An autocatalysis was observed by one of the products formed, that is, Cu (II). A 1:2 stoichiometry (DG : DPC) exhibited reaction between the DG and DPC in an aqueous alkaline medium. The reaction was of first order in [DPC] when [DPC] < < [DG], while the order with respect to [DG] and [OH−] was less than unity, whereas periodate had retarding effect on the rate of reaction. Ionic strength had a negligible effect on the rate of reaction. The main reaction products were identified by the spot tests and spectroscopic analysis. The product, Cu (II), catalyzed the reaction with a fractional order. A composite mechanism involving uncatalyzed and autocatalyzed reaction paths was proposed. The activation parameters with respect to slow step of the mechanism and also the thermodynamic quantities were determined and discussed.
中文翻译:

碱性高碘酸铜(III)络合物对d-葡萄糖醇的自催化氧化:动力学和机理方法
的自催化氧化d在0.06摩尔分米的恒定离子强度在含水碱性介质中-glucitol(DG)由diperiodatocuprate(III)(DPC)-3分光光度研究。所形成的产物之一,即Cu(II)观察到自催化作用。化学计量比为1:2(DG:DPC)表示DG和DPC在碱性水溶液中发生反应。反应第一阶中[DPC]时[DPC] <<[DG],而相对于以[DG]和[OH -]小于1,而高碘酸盐对反应速率具有阻滞作用。离子强度对反应速率的影响可忽略不计。主要反应产物通过现场测试和光谱分析鉴定。产物Cu(II)以分数级催化反应。提出了涉及未催化和自催化反应路径的复合机理。确定并讨论了关于机构慢速步进的激活参数以及热力学量。
更新日期:2020-11-15
中文翻译:

碱性高碘酸铜(III)络合物对d-葡萄糖醇的自催化氧化:动力学和机理方法
的自催化氧化d在0.06摩尔分米的恒定离子强度在含水碱性介质中-glucitol(DG)由diperiodatocuprate(III)(DPC)-3分光光度研究。所形成的产物之一,即Cu(II)观察到自催化作用。化学计量比为1:2(DG:DPC)表示DG和DPC在碱性水溶液中发生反应。反应第一阶中[DPC]时[DPC] <<[DG],而相对于以[DG]和[OH -]小于1,而高碘酸盐对反应速率具有阻滞作用。离子强度对反应速率的影响可忽略不计。主要反应产物通过现场测试和光谱分析鉴定。产物Cu(II)以分数级催化反应。提出了涉及未催化和自催化反应路径的复合机理。确定并讨论了关于机构慢速步进的激活参数以及热力学量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号