当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization of Metal Single Atoms on Carbon and TiO2 Supports for CO2 Hydrogenation: The Importance of Regulating Charge Transfer
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-11-16 , DOI: 10.1002/admi.202001777 Camila Rivera‐Cárcamo 1 , Canio Scarfiello 1 , Ana B. García 2 , Yann Tison 3 , Hervé Martinez 3 , Walid Baaziz 4 , Ovidiu Ersen 4 , Carole Le Berre 1 , Philippe Serp 1
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-11-16 , DOI: 10.1002/admi.202001777 Camila Rivera‐Cárcamo 1 , Canio Scarfiello 1 , Ana B. García 2 , Yann Tison 3 , Hervé Martinez 3 , Walid Baaziz 4 , Ovidiu Ersen 4 , Carole Le Berre 1 , Philippe Serp 1
Affiliation
|
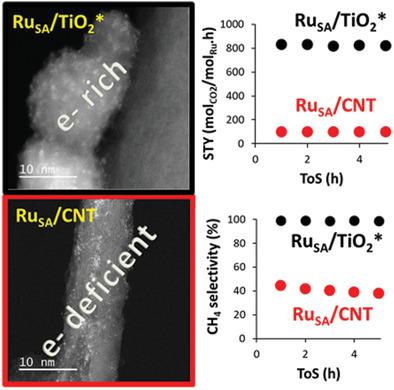
Supported single atoms constitute excellent models for understanding heterogenous catalysis and have achieved breakthroughs during the past years. How to prevent the aggregation and modulate activity of these species via metal‐support interaction should be considered for practical applications. This work presents simple methods involving the creation of carbon‐ (on carbon nanotube (CNT)) or oxygen‐vacancies (on TiO2) to stabilize nickel and ruthenium single atoms. The defective supports and the resulting catalysts are characterized by a large variety of techniques. These analyses show that this strategy is efficient for the preparation of ultra‐dispersed catalysts. Comparison of the catalytic performances of these catalysts for the CO2 hydrogenation reaction is also reported. Catalysts supported on TiO2 are more active and sometimes more stable than those deposited on CNT. Nickel catalysts are very selective for the production of CO, and ruthenium catalysts are more selective for the production of methane. Most importantly, it is shown that, in the case of Ru, a direct correlation exists between the electronic density on the metal and the selectivity; electron‐rich species produce selectively methane, while electron‐deficient species orientate the selectivity toward CO. This work may figure a new way for the synthesis of ultra‐dispersed catalysts for various applications.
中文翻译:

碳和TiO2载体上金属单原子在CO2加氢中的稳定作用:调节电荷转移的重要性
负载的单原子构成了理解多相催化的出色模型,并且在过去几年中取得了突破。在实际应用中应考虑如何通过金属-载体相互作用防止这些物种的聚集和调节活性。这项工作提出了简单的方法,涉及创建碳(在碳纳米管(CNT)上)或氧空位(在TiO 2上)来稳定镍和钌单原子。缺陷载体和所得催化剂的特征在于多种技术。这些分析表明,该策略对于制备超分散催化剂是有效的。这些催化剂对CO 2的催化性能比较还报道了氢化反应。负载在TiO 2上的催化剂比沉积在CNT上的催化剂更具活性,有时甚至更稳定。镍催化剂对生产CO的选择性很高,而钌催化剂对生产甲烷的选择性更高。最重要的是,证明了在Ru的情况下,金属上的电子密度与选择性之间存在直接的相关性。富含电子的物质选择性地产生甲烷,而缺乏电子的物质则使对CO的选择性定向。这项工作可能为合成用于各种应用的超分散催化剂提供了一种新途径。
更新日期:2020-11-16
中文翻译:

碳和TiO2载体上金属单原子在CO2加氢中的稳定作用:调节电荷转移的重要性
负载的单原子构成了理解多相催化的出色模型,并且在过去几年中取得了突破。在实际应用中应考虑如何通过金属-载体相互作用防止这些物种的聚集和调节活性。这项工作提出了简单的方法,涉及创建碳(在碳纳米管(CNT)上)或氧空位(在TiO 2上)来稳定镍和钌单原子。缺陷载体和所得催化剂的特征在于多种技术。这些分析表明,该策略对于制备超分散催化剂是有效的。这些催化剂对CO 2的催化性能比较还报道了氢化反应。负载在TiO 2上的催化剂比沉积在CNT上的催化剂更具活性,有时甚至更稳定。镍催化剂对生产CO的选择性很高,而钌催化剂对生产甲烷的选择性更高。最重要的是,证明了在Ru的情况下,金属上的电子密度与选择性之间存在直接的相关性。富含电子的物质选择性地产生甲烷,而缺乏电子的物质则使对CO的选择性定向。这项工作可能为合成用于各种应用的超分散催化剂提供了一种新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号