European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-11-16 , DOI: 10.1016/j.ejmech.2020.113021 Dries De Ruysscher , Luping Pang , Stijn M.G. Lenders , Davie Cappoen , Paul Cos , Jef Rozenski , Sergei V. Strelkov , Stephen D. Weeks , Arthur Van Aerschot
|
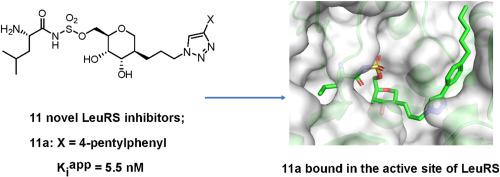
Leucyl-tRNA synthetase (LeuRS) is a clinically validated target for the development of antimicrobials. This enzyme catalyzes the formation of charged tRNALeu molecules, an essential substrate for protein translation. In the first step of catalysis LeuRS activates leucine using ATP, forming a leucyl-adenylate intermediate. Bi-substrate inhibitors that mimic this chemically labile phosphoanhydride-linked nucleoside have proven to be potent inhibitors of different members of the aminoacyl tRNA synthetase family but, too date, they have demonstrated poor antibacterial activity. We synthesized a small series of 1,5-anhydrohexitol based analogues coupled to a variety of triazoles and performed detailed structure-activity relationship studies with bacterial LeuRS. In an in vitro assay, values in the nanomolar range were demonstrated. Inhibitory activity differences between the compounds revealed that the polarity and size of the triazole substituents affect binding. X-ray crystallographic studies of N. gonorrhoeae LeuRS in complex with all the inhibitors highlighted the crucial interactions defining their relative enzyme inhibitory activities. We further examined their in vitro antimicrobial properties by screening against several bacterial and yeast strains. While only weak antibacterial activity against M. tuberculosis was detected, the extensive structural data which were obtained could make these LeuRS inhibitors a suitable starting point towards further antibiotic development.
中文翻译:

新型基于脱水己糖醇的亮氨酰tRNA合成酶抑制剂的合成及结构活性研究
Leucyl-tRNA合成酶(LeuRS)是抗微生物药物开发的临床验证靶标。这种酶催化带电荷的tRNA Leu分子的形成,这是蛋白质翻译的重要底物。在催化的第一步中,LeuRS使用ATP激活亮氨酸,形成亮氨酰-腺苷酸中间体。模仿这种化学上不稳定的磷酸酐连接的核苷的双底物抑制剂已被证明是氨酰基tRNA合成酶家族不同成员的有效抑制剂,但迄今为止,它们也显示出较差的抗菌活性。我们合成了少量的基于1,5-脱水己糖醇的类似物,并与多种三唑偶联,并使用细菌LeuRS进行了详细的结构-活性关系研究。在体外测定中证明了在纳摩尔范围内的值。化合物之间的抑制活性差异表明三唑取代基的极性和大小会影响结合。淋病奈瑟氏球菌LeuRS与所有抑制剂的复合物的X射线晶体学研究突显了定义其相对酶抑制活性的关键相互作用。我们通过针对几种细菌和酵母菌株进行筛选进一步检查了它们的体外抗菌性能。虽然仅检测到了对结核分枝杆菌的弱抗菌活性,但获得的大量结构数据可使这些LeuRS抑制剂成为进一步开发抗生素的合适起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号