Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2020-11-16 , DOI: 10.1007/s10593-020-02817-y Mikhail A. Nazarov , Irina A. Tolmacheva , Daria V. Eroshenko , Olga A. Maiorova , Maksim V. Dmitriev , Victoria V. Grishko
|
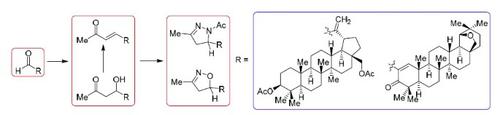
α,β-Unsaturated lupane and 19β,28-epoxy-18α-oleanane aldehydes were used in the synthesis of triterpenoids bearing substituted 1,2-azole moieties (1-acetyl-3-methyl-4,5-dihydro-1H-pyrazole and 3-methyl-4,5-dihydroisoxazole) at the rings А and Е. The route of synthesis for these 1,2-azole derivatives of triterpenes included an aldol condensation of α,β-unsaturated aldehydes with acetone, the products of which (α,β-unsaturated methyl ketone and β-hydroxy ketone) underwent a further cycloaddition reaction with acetylhydrazide and hydroxylamine. Cytotoxic activity studies of the synthesized compounds against seven cancer cell lines (Hep-2, HCT116, MS, RD TE32, A549, MCF-7, and PC-3) showed that the highest cytotoxicity (IC50 0.66–11.97 μM) against all tested cell lines was exihbited by 19β,28-epoxy-18α-oleanane aldehyde and the products of its condensation reactions with acetone and acetylhydrazide.
中文翻译:

在α,β-不饱和三萜醛的基础上合成1,2-唑衍生物
α,β不饱和羽扇烷和19β,28-环氧- 18α -齐墩果烷醛是在三萜类化合物的合成中使用轴承取代的1,2-唑部分(1-乙酰基-3-甲基-4,5-二氢- 1 H ^ -吡咯和3-甲基-4,5-二氢异恶唑)在А和Å环上。这些三萜的1,2-唑衍生物的合成路线包括α,β-不饱和醛与丙酮的醛醇缩合,其产物(α,β-不饱和甲基酮和β-羟基酮)经过进一步的环加成反应与乙酰肼和羟胺反应。合成化合物对7种癌细胞系(Hep-2,HCT116,MS,RD TE32,A549,MCF-7和PC-3)的细胞毒活性研究表明,最高的细胞毒性(IC 50 19β,28-环氧-18α-油烷醛及其与丙酮和乙酰肼的缩合反应产物显示出针对所有测试细胞系的0.66–11.97μM)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号