当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
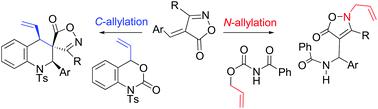
N-Allylation versus C-allylation of intermediates from aza-Michael adducts of arylideneisoxazol-5-ones
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-05 , DOI: 10.1039/d0ob01998d Shu-Hao Wan, Xuan-An Li, Yi-Hung Liu, Shiuh-Tzung Liu
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-11-05 , DOI: 10.1039/d0ob01998d Shu-Hao Wan, Xuan-An Li, Yi-Hung Liu, Shiuh-Tzung Liu
|
Reactions of arylidene-isoxazol-5-ones with intermediates from palladium-catalysed decarboxylation of allyl carbamates proceeded through aza-Michael addition and N-allylation to give the corresponding bis-adducts, β-amido-N-allylated products, in good yields. In similar reactions with 4-vinyl-1,4-dihydro-2H-3,1-benzoxazin-2-one, a cyclic allyl carbamate, C-allylation took place to yield a series of spiro[isoxazole-4,3′-quinolin]-5-ones in high yields. Regio-selective N- versus C-allylation is illustrated to occur in an inter- versus intra-molecular fashion. The structure and stereochemistry of these products are determined by NMR spectroscopy and further confirmed by X-ray crystallography. This work offers an excellent method for the preparation of various substituted isoxazol-5-ones.
中文翻译:

亚芳基异恶唑-5-酮氮杂-迈克尔加合物中间体的N-烯丙基化与C-烯丙基化
亚芳基-异恶唑-5-酮与钯催化的烯丙基氨基甲酸酯脱羧中间体的反应通过氮杂迈克尔加成和N-烯丙基化得到相应的双加合物,即β-酰胺基-N-烯丙基化产物,收率良好。在与环状氨基甲酸烯丙酯 4-vinyl-1,4-dihydro-2 H -3,1-benzoxazin-2-one 的类似反应中,发生C-烯丙基化,生成一系列螺[isoxazole-4,3' -quinolin]-5-ones 的高产率。区域选择性N -与 C -烯丙基化发生在分子内时尚。这些产品的结构和立体化学由 NMR 光谱确定,并通过 X 射线晶体学进一步证实。这项工作为制备各种取代的异恶唑-5-酮提供了一种极好的方法。
更新日期:2020-11-15
中文翻译:

亚芳基异恶唑-5-酮氮杂-迈克尔加合物中间体的N-烯丙基化与C-烯丙基化
亚芳基-异恶唑-5-酮与钯催化的烯丙基氨基甲酸酯脱羧中间体的反应通过氮杂迈克尔加成和N-烯丙基化得到相应的双加合物,即β-酰胺基-N-烯丙基化产物,收率良好。在与环状氨基甲酸烯丙酯 4-vinyl-1,4-dihydro-2 H -3,1-benzoxazin-2-one 的类似反应中,发生C-烯丙基化,生成一系列螺[isoxazole-4,3' -quinolin]-5-ones 的高产率。区域选择性N -与 C -烯丙基化发生在分子内时尚。这些产品的结构和立体化学由 NMR 光谱确定,并通过 X 射线晶体学进一步证实。这项工作为制备各种取代的异恶唑-5-酮提供了一种极好的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号