当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formulation and clinical translation of [177Lu]Lu-trastuzumab for radioimmunotheranostics of metastatic breast cancer
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-11-14 , DOI: 10.1039/d0md00319k Mohini Guleria 1 , Rohit Sharma 1 , Jeyachitra Amirdhanayagam 1 , Haladhar D Sarma 2 , Venkatesh Rangarajan 3 , Ashutosh Dash 1, 4 , Tapas Das 1, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2020-11-14 , DOI: 10.1039/d0md00319k Mohini Guleria 1 , Rohit Sharma 1 , Jeyachitra Amirdhanayagam 1 , Haladhar D Sarma 2 , Venkatesh Rangarajan 3 , Ashutosh Dash 1, 4 , Tapas Das 1, 4
Affiliation
|
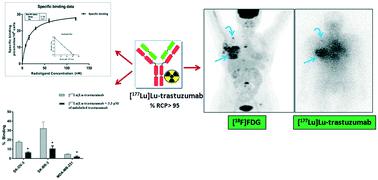
Trastuzumab (Herceptin®) is an approved immunotherapeutic agent used for the treatment of metastatic breast cancer over-expressing HER2 antigen receptors. The aim of the present work is to standardize the formulation protocol of [177Lu]Lu-trastuzumab addressing various reaction parameters, evaluating the efficacy of the radiolabeled product by in vitro investigations, scaling-up the preparation for administration in patients and performing preliminary clinical studies in patients suffering from metastatic breast cancer. Trastuzumab was conjugated with a suitable bi-functional chelating agent namely, p-NCS-benzyl-DOTA. On average 6.15 ± 0.92 p-NCS-benzyl-DOTA molecules were observed to be attached to each trastuzumab moiety. [177Lu]Lu-trastuzumab could be prepared with >95% radiochemical purity (% RCP) employing the optimized radiolabeling procedure. In vitro studies revealed the affinity of [177Lu]Lu-trastuzumab towards HER2 +ve cancer cell lines as well as against HER2 protein (Kd = 13.61 nM and 11.36 nM, respectively). The value for percentage immunoreactive fraction (% IRF) for [177Lu]Lu-trastuzumab was observed to be 76.92 ± 2.80. Bio-distribution studies in Swiss mice revealed non-specific uptake in the blood, liver, lungs and heart followed by gradual clearance of activity predominantly through the hepatobiliary route. Preliminary clinical studies carried out in 8 cancer patients with immunohistochemically proven HER2 positive metastatic breast cancer revealed preferential localization of [177Lu]Lu-trastuzumab in breast cancer lesions, which was in concordance with [18F]FDG-PET scans recorded earlier in the same patient indicating the potential of the agent towards radioimmunotheranostic applications.
中文翻译:

用于转移性乳腺癌放射免疫治疗的[177Lu]Lu-曲妥珠单抗的配方及临床转化
曲妥珠单抗 (Herceptin®) 是一种批准的免疫治疗药物,用于治疗过度表达 HER2 抗原受体的转移性乳腺癌。目前工作的目的是标准化 [ 177 Lu]Lu-曲妥珠单抗的配制方案,解决各种反应参数,通过体外研究评估放射性标记产品的功效,扩大患者给药制剂并进行初步临床对患有转移性乳腺癌的患者进行的研究。曲妥珠单抗与合适的双功能螯合剂(即对-NCS-苄基-DOTA)缀合。观察到平均 6.15 ± 0.92 个p -NCS-苄基-DOTA 分子附着到每个曲妥珠单抗部分。使用优化的放射性标记程序,可以制备具有 >95% 放射化学纯度 (% RCP) 的[ 177 Lu]Lu-曲妥珠单抗。体外研究揭示了[ 177 Lu]Lu-曲妥珠单抗对HER2 +ve 癌细胞系以及对HER2蛋白的亲和力(K d分别= 13.61 nM和11.36 nM)。观察到[ 177 Lu]Lu-曲妥珠单抗的免疫反应分数百分比(%IRF)值为76.92±2.80。对瑞士小鼠的生物分布研究显示,在血液、肝脏、肺和心脏中非特异性摄取,随后主要通过肝胆途径逐渐清除活性。对 8 名免疫组织化学证实的 HER2 阳性转移性乳腺癌患者进行的初步临床研究显示,[ 177 Lu]Lu-曲妥珠单抗优先定位于乳腺癌病灶中,这与早期记录的[ 18 F]FDG-PET 扫描一致。同一名患者表明该药物在放射免疫治疗应用方面具有潜力。
更新日期:2021-01-12
中文翻译:

用于转移性乳腺癌放射免疫治疗的[177Lu]Lu-曲妥珠单抗的配方及临床转化
曲妥珠单抗 (Herceptin®) 是一种批准的免疫治疗药物,用于治疗过度表达 HER2 抗原受体的转移性乳腺癌。目前工作的目的是标准化 [ 177 Lu]Lu-曲妥珠单抗的配制方案,解决各种反应参数,通过体外研究评估放射性标记产品的功效,扩大患者给药制剂并进行初步临床对患有转移性乳腺癌的患者进行的研究。曲妥珠单抗与合适的双功能螯合剂(即对-NCS-苄基-DOTA)缀合。观察到平均 6.15 ± 0.92 个p -NCS-苄基-DOTA 分子附着到每个曲妥珠单抗部分。使用优化的放射性标记程序,可以制备具有 >95% 放射化学纯度 (% RCP) 的[ 177 Lu]Lu-曲妥珠单抗。体外研究揭示了[ 177 Lu]Lu-曲妥珠单抗对HER2 +ve 癌细胞系以及对HER2蛋白的亲和力(K d分别= 13.61 nM和11.36 nM)。观察到[ 177 Lu]Lu-曲妥珠单抗的免疫反应分数百分比(%IRF)值为76.92±2.80。对瑞士小鼠的生物分布研究显示,在血液、肝脏、肺和心脏中非特异性摄取,随后主要通过肝胆途径逐渐清除活性。对 8 名免疫组织化学证实的 HER2 阳性转移性乳腺癌患者进行的初步临床研究显示,[ 177 Lu]Lu-曲妥珠单抗优先定位于乳腺癌病灶中,这与早期记录的[ 18 F]FDG-PET 扫描一致。同一名患者表明该药物在放射免疫治疗应用方面具有潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号