当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption capacity of the corrosion products of nanoscale zerovalent iron for emerging contaminants
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0en00886a Junmin Deng 1, 2, 3, 4, 5 , Sungjun Bae 6, 7, 8, 9 , Sunho Yoon 6, 7, 8, 9 , Mathieu Pasturel 1, 3, 5, 10, 11 , Rémi Marsac 1, 3, 11, 12, 13 , Khalil Hanna 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0en00886a Junmin Deng 1, 2, 3, 4, 5 , Sungjun Bae 6, 7, 8, 9 , Sunho Yoon 6, 7, 8, 9 , Mathieu Pasturel 1, 3, 5, 10, 11 , Rémi Marsac 1, 3, 11, 12, 13 , Khalil Hanna 1, 2, 3, 4, 5
Affiliation
|
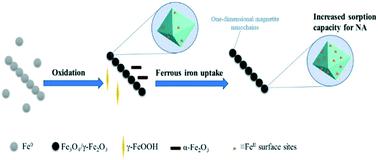
Despite the extensive use of nanoscale zerovalent iron (NZVI) in water and soil remediation, no data exist on the reactivity of secondary iron minerals formed upon the NZVI corrosion. Herein, we investigated the oxidation kinetics of NZVI by monitoring the variations of pH, oxidation–reduction potential (ORP) and dissolved Fe(II) concentration, and then examined the reactivity of resulting oxidized particles for the adsorption of an emerging contaminant (nalidixic acid (NA)). NA adsorption was found the greatest on oxidized particles and negligible on the fresh NZVI. Interestingly, the formed secondary mineral phases exhibited an unusual pH adsorption curve with an unexpected great adsorption at alkaline pH values. X-ray photoelectron spectroscopy and high resolution-transmission electron microscopy revealed a gradual increase in the Fe(II) content at the surface of the magnetite phase over the reaction time. Additional experiments and surface complexation modeling showed that the enhanced adsorption of NA onto the secondary magnetite is due to the formation of surface bound Fe(II). Fe(II) is released into the solution because, for instance, of the presence of organic buffer molecules, decreased surface Fe(II) and NA adsorption at alkaline pH values. This work sheds light on an overseen aspect of the reactivity of secondary iron minerals resulting from NZVI passivation, which can bind co-existing emerging contaminants and then affect their fate in the environment.
中文翻译:

纳米零价铁腐蚀产物对新兴污染物的吸附能力
尽管在水和土壤修复中广泛使用了纳米级零价铁(NZVI),但尚无关于NZVI腐蚀后形成的次生铁矿物质反应性的数据。在这里,我们通过监测pH,氧化还原电位(ORP)和溶解的Fe(II)的变化研究了NZVI的氧化动力学。浓度),然后检查所得氧化颗粒对新出现的污染物(纳立二酸(NA))吸附的反应性。发现NA吸附在氧化颗粒上最大,而在新鲜NZVI上可忽略不计。有趣的是,形成的次生矿物相显示出异常的pH吸附曲线,在碱性pH值下具有出乎意料的巨大吸附。X射线光电子能谱和高分辨率透射电子显微镜显示随着反应时间,磁铁矿相表面的Fe(II)含量逐渐增加。额外的实验和表面络合模型表明,NA在次级磁铁矿上的吸附增强是由于表面结合的Fe(II)的形成。铁(II)被释放到溶液中,因为例如存在有机缓冲分子,在碱性pH值下表面Fe(II)减少和NA吸附。这项工作揭示了由NZVI钝化产生的次生铁矿物质反应性的可监督方面,该物质可结合共存的新兴污染物,然后影响其在环境中的命运。
更新日期:2020-11-15
中文翻译:

纳米零价铁腐蚀产物对新兴污染物的吸附能力
尽管在水和土壤修复中广泛使用了纳米级零价铁(NZVI),但尚无关于NZVI腐蚀后形成的次生铁矿物质反应性的数据。在这里,我们通过监测pH,氧化还原电位(ORP)和溶解的Fe(II)的变化研究了NZVI的氧化动力学。浓度),然后检查所得氧化颗粒对新出现的污染物(纳立二酸(NA))吸附的反应性。发现NA吸附在氧化颗粒上最大,而在新鲜NZVI上可忽略不计。有趣的是,形成的次生矿物相显示出异常的pH吸附曲线,在碱性pH值下具有出乎意料的巨大吸附。X射线光电子能谱和高分辨率透射电子显微镜显示随着反应时间,磁铁矿相表面的Fe(II)含量逐渐增加。额外的实验和表面络合模型表明,NA在次级磁铁矿上的吸附增强是由于表面结合的Fe(II)的形成。铁(II)被释放到溶液中,因为例如存在有机缓冲分子,在碱性pH值下表面Fe(II)减少和NA吸附。这项工作揭示了由NZVI钝化产生的次生铁矿物质反应性的可监督方面,该物质可结合共存的新兴污染物,然后影响其在环境中的命运。











































 京公网安备 11010802027423号
京公网安备 11010802027423号