当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vivo Biogenesis of a De Novo Designed Iron–Sulfur Protein
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-13 , DOI: 10.1021/acssynbio.0c00514 Bhanu P. Jagilinki 1, 2 , Stefan Ilic 3 , Cristian Trncik 4 , Alexei M. Tyryshkin 1 , Douglas H. Pike 1 , Wolfgang Lubitz 5 , Eckhard Bill 5 , Oliver Einsle 4 , James A. Birrell 5 , Barak Akabayov 3 , Dror Noy 2 , Vikas Nanda 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-13 , DOI: 10.1021/acssynbio.0c00514 Bhanu P. Jagilinki 1, 2 , Stefan Ilic 3 , Cristian Trncik 4 , Alexei M. Tyryshkin 1 , Douglas H. Pike 1 , Wolfgang Lubitz 5 , Eckhard Bill 5 , Oliver Einsle 4 , James A. Birrell 5 , Barak Akabayov 3 , Dror Noy 2 , Vikas Nanda 1
Affiliation
|
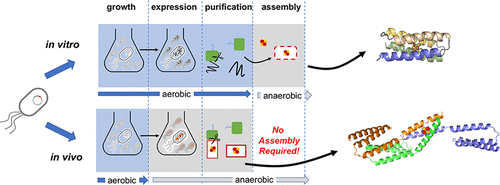
In vivo expression of metalloproteins requires specific metal trafficking and incorporation machinery inside the cell. Synthetic designed metalloproteins are typically purified without the target metal, which is subsequently introduced through in vitro reconstitution. The extra step complicates protein optimization by high-throughput library screening or laboratory evolution. We demonstrate that a designed coiled-coil iron–sulfur protein (CCIS) assembles robustly with [4Fe-4S] clusters in vivo. While in vitro reconstitution produces a mixture of oligomers that depends on solution conditions, in vivo production generates a stable homotrimer coordinating a single, diamagnetic [4Fe-4S]2+ cluster. The multinuclear cluster of in vivo assembled CCIS is more resistant to degradation by molecular oxygen. Only one of the two metal coordinating half-sites is required in vivo, indicating specificity of molecular recognition in recruitment of the metal cluster. CCIS, unbiased by evolution, is a unique platform to examine iron–sulfur protein biogenesis and develop synthetic multinuclear oxidoreductases.
中文翻译:

体内一的生物合成从头设计的铁硫蛋白
金属蛋白的体内表达需要细胞内特定的金属运输和整合机制。通常在没有目标金属的情况下纯化合成设计的金属蛋白,然后通过体外重组将其引入。通过高通量文库筛选或实验室进化,额外的步骤使蛋白质优化变得复杂。我们证明了一种设计的盘绕线圈铁-硫蛋白(CCIS)在体内与[4Fe-4S]簇牢固地组装在一起。而在体外重组产生依赖于溶液条件,低聚物的混合物在体内的生产产生了稳定的同源三聚体配位的单个,反磁性[的4Fe-4S] 2+簇。体内组装的CCIS的多核簇更耐分子氧降解。在体内仅需要两个金属配位半位中的一个,表明在金属簇募集中分子识别的特异性。CCIS,不受进化的偏见,是一个独特的平台,可以检查铁硫蛋白的生物发生并开发合成的多核氧化还原酶。
更新日期:2020-12-18
中文翻译:

体内一的生物合成从头设计的铁硫蛋白
金属蛋白的体内表达需要细胞内特定的金属运输和整合机制。通常在没有目标金属的情况下纯化合成设计的金属蛋白,然后通过体外重组将其引入。通过高通量文库筛选或实验室进化,额外的步骤使蛋白质优化变得复杂。我们证明了一种设计的盘绕线圈铁-硫蛋白(CCIS)在体内与[4Fe-4S]簇牢固地组装在一起。而在体外重组产生依赖于溶液条件,低聚物的混合物在体内的生产产生了稳定的同源三聚体配位的单个,反磁性[的4Fe-4S] 2+簇。体内组装的CCIS的多核簇更耐分子氧降解。在体内仅需要两个金属配位半位中的一个,表明在金属簇募集中分子识别的特异性。CCIS,不受进化的偏见,是一个独特的平台,可以检查铁硫蛋白的生物发生并开发合成的多核氧化还原酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号