Water Research ( IF 11.4 ) Pub Date : 2020-11-15 , DOI: 10.1016/j.watres.2020.116643 Junzhi Zhang , Yu Liao , Qi Wang , Chunmiao Wang , Jianwei Yu
|
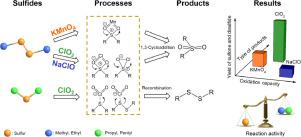
Swampy/septic odor caused by various sulfides is one of the most frequently encountered odor problems in drinking water. However, even though it is much more offensive, few studies have specifically focused on swampy/septic odor compared to the extensively studied musty/earthy problems. In this work, four sulfide odorants, diamyl sulfide (DAS), dipropyl sulfide (DPS), dimethyl disulfide (DMDS) and diethyl disulfide (DEDS), were selected to evaluate the treatment performance of different oxidation processes in drinking water. The results demonstrated that DMDS, DEDS, DPS and DAS could be oxidized effectively by KMnO4, NaClO and ClO2. The oxidation processes could be well described by the second-order kinetic model, in which k values of selected sulfides followed the order DMDS≈DEDS ≪ DPS≈DAS. As for the three oxidants, the order of reactivity was KMnO4 ≪ ClO2 < NaClO, which was also verified in raw water. The results of oxidation treatability, reaction kinetics and mechanisms confirmed that the characteristics of the central sulfur atom rather than the side chain is the decisive factor in controlling the oxidation rate and transformation pathway of sulfides. The transformation products and pathways were significantly different for the three oxidants. Sulfones (DPSO, DASO) were always formed by cycloaddition reactions during KMnO4 oxidation, yet recombination reactions proceeded during ClO2 oxidation and formed more products, such as MADS, DADS and EADS. Density functional theory (DFT) calculations confirmed that the differences in transformation pathways were caused by the variations in the activity of the oxidants and sulfides. Finally, NaClO was certified as the most effective oxidant for controlling sulfide odorants in drinking water treatment.
中文翻译:

饮用水中不同氧化过程降解臭味硫化物的性能,反应动力学和机理
由各种硫化物引起的沼泽/腐臭是饮用水中最常见的气味问题之一。然而,尽管它更具攻击性,但与广泛研究的霉味/耳垢问题相比,很少有研究专门针对沼泽/化脓性气味。在这项工作中,选择了四种硫化物加味剂:二戊基硫醚(DAS),二丙基硫醚(DPS),二甲基二硫醚(DMDS)和二乙基二硫醚(DEDS),以评估饮用水中不同氧化过程的处理性能。结果表明DMDS,DEDS,DPS和DAS可以被KMnO 4, NaClO和ClO 2有效地氧化。氧化过程可以用二阶动力学模型很好地描述,其中k选定硫化物的值遵循DMDS≈DEDS≪DPS≈DAS的顺序。至于三个氧化剂,反应性的顺序为高锰酸钾4 «CLO 2 <次氯酸钠,这也是在原水验证。氧化处理性,反应动力学和机理的结果证实,中心硫原子而不是侧链的特征是控制硫化物的氧化速率和转化途径的决定性因素。三种氧化剂的转化产物和途径明显不同。砜(DPSO,DASO)总是在KMnO 4氧化过程中通过环加成反应形成,而在ClO 2过程中仍会发生重组反应氧化并形成更多的产品,例如MADS,DADS和EADS。密度泛函理论(DFT)计算证实,转化途径的差异是由氧化剂和硫化物活性的变化引起的。最终,NaClO被证明是控制饮用水处理中硫化物气味的最有效氧化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号