Neuroscience ( IF 2.9 ) Pub Date : 2020-11-14 , DOI: 10.1016/j.neuroscience.2020.10.025 Stephen Beesley 1 , Thomas Sullenberger 1 , Roshan Ailani 1 , Cameron D'Orio 1 , Mathew S Crockett 1 , Sanjay S Kumar 1
|
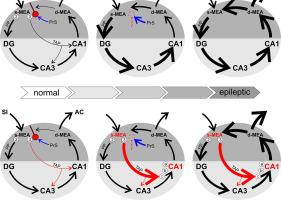
Entrainment of the hippocampus by the medial entorhinal area (MEA) in Temporal Lobe Epilepsy (TLE), the most common type of drug-resistant epilepsy in adults, is believed to be mediated primarily through the perforant pathway (PP), which connects stellate cells in layer (L) II of the MEA with granule cells of the dentate gyrus (DG) to drive the hippocampal tri-synaptic circuit. Using immunohistochemistry, high-resolution confocal microscopy and the rat pilocarpine model of TLE, we show here that the lesser known temporoammonic pathway (TAP) plays a significant role in transferring MEA pathology to the CA1 region of the hippocampus independently of the PP. The pathology observed was region-specific and restricted primarily to the CA1c subfield of the hippocampus. As shown previously, daily intracranial infusion of D-serine (100 μm), an antagonist of GluN3-containing triheteromeric N-Methyl D-aspartate receptors (t-NMDARs), into the MEA prevented loss of LIII neurons and epileptogenesis. This intervention in the MEA led to the rescue of hippocampal CA1 neurons that would have otherwise perished in the epileptic animals, and down regulation of the expression of astrocytes and microglia thereby mitigating the effects of neuroinflammation. Interestingly, these changes were not observed to a similar extent in other regions of vulnerability like the hilus, DG or CA3, suggesting that the pathology manifest in CA1 is driven predominantly through the TAP. This work highlights TAP’s role in the entrainment of the hippocampus and identifies specific areas for therapeutic intervention in dealing with TLE.
中文翻译:

D-丝氨酸对内侧内嗅区的干预通过颞氨通路改变 CA1 海马中 TLE 相关的病理学
颞叶癫痫 (TLE) 是成人最常见的耐药性癫痫类型,内侧内嗅区 (MEA) 对海马的夹带被认为主要是通过连接星状细胞的穿通通路(PP) 介导的MEA 的第二层 (L) 与齿状回 (DG) 的颗粒细胞一起驱动海马三突触回路。使用免疫组织化学、高分辨率共聚焦显微镜和 TLE 的大鼠毛果芸香碱模型,我们在此表明鲜为人知的颞氨通路(TAP) 在将 MEA 病理学独立于 PP 转移到海马 CA1 区方面发挥着重要作用。观察到的病理学具有区域特异性,主要局限于海马的CA1c亚区。如前所述,每天向 MEA 颅内输注 D-丝氨酸(100 μm)(一种含有 GluN3 的三异聚 N-甲基 D-天冬氨酸受体 ( t -NMDAR) 的拮抗剂)可防止 LIII 神经元的损失和癫痫发生。这种对 MEA 的干预拯救了癫痫动物中可能会死亡的海马 CA1 神经元,并下调了星形胶质细胞和小胶质细胞的表达,从而减轻了神经炎症的影响。有趣的是,在其他脆弱区域(如门、DG 或 CA3)中没有观察到类似程度的这些变化,这表明 CA1 中表现的病理主要是通过 TAP 驱动的。这项工作强调了 TAP 在海马体牵引中的作用,并确定了治疗 TLE 的治疗干预的特定领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号