Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-11-14 , DOI: 10.1016/j.jmb.2020.11.012 Arianna Barchiesi , Veronica Bazzani , Vanessa Tolotto , Praveenraj Elancheliyan , Michał Wasilewski , Agnieszka Chacinska , Carlo Vascotto
|
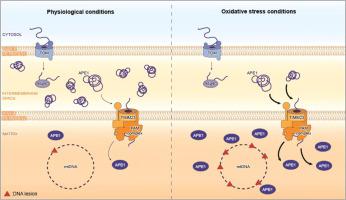
Mitochondria are essential cellular organelles that import the majority of proteins to sustain their function in cellular metabolism and homeostasis. Due to their role in oxidative phosphorylation, mitochondria are constantly affected by oxidative stress. Stability of mitochondrial DNA (mtDNA) is essential for mitochondrial physiology and cellular well-being and for this reasons mtDNA lesions have to be rapidly recognized and repaired. Base excision repair (BER) is the main pathway responsible for repair non-helix distorting base lesions both into the nucleus and in mitochondria. Apurinic/Apyrimidinic Endonuclease 1 (APE1) is a key component of BER pathway and the only protein that can recognize and process an abasic (AP) site. Comprehensions of the mechanisms regulating APE1 intracellular trafficking are still fragmentary. In this study we focused our attention on the mitochondrial form of APE1 protein and how oxidative stress induce its translocation to maintain mtDNA integrity. Our data proved that: (i) the rise of mitochondrial ROS determines a very rapid translocation of APE1 from the intermembrane space (IMS) into the matrix; and (ii) TIM23/PAM machinery complex is responsible for the matrix translocation of APE1. Moreover, our data support the hypothesis that the IMS, were the majority of APE1 resides, could represent a sort of storage site for the protein.
中文翻译:

线粒体氧化应激通过TIM23复合物诱导Apurinic / apyrimidinic内切核酸酶1蛋白的快速膜间空间/基质移位
线粒体是必需的细胞器,其导入大多数蛋白质以维持其在细胞代谢和体内平衡中的功能。由于线粒体在氧化磷酸化中的作用,因此经常受到氧化应激的影响。线粒体DNA(mtDNA)的稳定性对于线粒体生理学和细胞健康至关重要,因此,必须迅速识别和修复mtDNA损伤。碱基切除修复(BER)是负责修复非螺旋扭曲的基本病变进入细胞核和线粒体的主要途径。尖酸/无嘧啶核糖核酸内切酶1(APE1)是BER途径的关键组成部分,也是唯一可以识别和处理脱碱基(AP)位点的蛋白质。调节APE1细胞内运输的机制的理解仍然是零碎的。在这项研究中,我们将注意力集中在线粒体形式的APE1蛋白上,以及氧化应激如何诱导其易位以维持mtDNA完整性。我们的数据证明:(i)线粒体ROS的增加决定了APE1从膜间空间(IMS)到基质的快速迁移;(ii)TIM23 / PAM机械联合体负责APE1的基质移位。此外,我们的数据支持以下假设:IMS是大多数APE1所驻留的,可能代表该蛋白质的一种存储位点。(ii)TIM23 / PAM机械联合体负责APE1的基质移位。此外,我们的数据支持以下假设:IMS是大多数APE1所驻留的,可能代表该蛋白质的一种存储位点。(ii)TIM23 / PAM机械联合体负责APE1的基质移位。此外,我们的数据支持以下假设:IMS是大多数APE1所驻留的,可能代表该蛋白质的一种存储位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号