当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the unique reactivity patterns of nickel/JoSPOphos manifold in the nickel-catalyzed enantioselective C–H cyclization of imidazoles
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-04 , DOI: 10.1039/d0sc04578k Jian-Biao Liu 1 , Xin Wang 1 , Antonis M Messinis 2 , Xiao-Jun Liu 1 , Rositha Kuniyil 2 , De-Zhan Chen 1 , Lutz Ackermann 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-04 , DOI: 10.1039/d0sc04578k Jian-Biao Liu 1 , Xin Wang 1 , Antonis M Messinis 2 , Xiao-Jun Liu 1 , Rositha Kuniyil 2 , De-Zhan Chen 1 , Lutz Ackermann 2
Affiliation
|
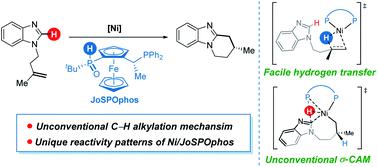
The 3d transition metal-catalyzed enantioselective C–H functionalization provides a sustainable strategy for the construction of chiral molecules. A better understanding of the catalytic nature of the reactions and the factors controlling the enantioselectivity is important for rational design of more efficient systems. Herein, the mechanisms of Ni-catalyzed enantioselective C–H cyclization of imidazoles are investigated by density functional theory (DFT) calculations. Both the π-allyl nickel(II)-promoted σ-complex-assisted metathesis (σ-CAM) and the nickel(0)-catalyzed oxidative addition (OA) mechanisms are disfavored. In addition to the typically proposed ligand-to-ligand hydrogen transfer (LLHT) mechanism, the reaction can also proceed via an unconventional σ-CAM mechanism that involves hydrogen transfer from the JoSPOphos ligand to the alkene through P–H oxidative addition/migratory insertion, C(sp2)–H activation via σ-CAM, and C–C reductive elimination. Importantly, computational results based on this new mechanism can indeed reproduce the experimentally observed enantioselectivities. Further, the catalytic activity of the π-allyl nickel(II) complex can be rationalized by the regeneration of the active nickel(0) catalyst via a stepwise hydrogen transfer, which was confirmed by experimental studies. The calculations reveal several significant roles of the secondary phosphine oxide (SPO) unit in JoSPOphos during the reaction. The improved mechanistic understanding will enable design of novel enantioselective C–H transformations.
中文翻译:

了解在镍催化的咪唑对映选择性C–H环化反应中,镍/ JoSPOphos歧管的独特反应模式
3d过渡金属催化的对映选择性C–H官能化为构建手性分子提供了可持续的策略。更好地理解反应的催化性质和控制对映选择性的因素对于合理设计更有效的系统非常重要。在本文中,通过密度泛函理论(DFT)计算研究了Ni催化的咪唑对映体C–H环化的机理。π-烯丙基镍(II)促进的σ络合物辅助复分解(σ-CAM)和镍(0)催化的氧化加成(OA)机理都是不利的。除了通常提出的配体到配体氢转移(LLHT)机理外,反应还可以通过一种非常规的σ-CAM机理,涉及氢从JoSPOphos配体通过P–H氧化加成/迁移插入,通过σ-CAM活化C(sp 2)–H以及C–C还原消除的氢转移。重要的是,基于这种新机制的计算结果确实可以重现实验观察到的对映选择性。此外,π烯丙基镍(催化活性II)配合物可以通过将活性镍(0)催化剂的再生合理化经由逐步的氢转移,已通过实验研究证实。该计算揭示了在反应过程中,JoSPOphos中次膦氧化物(SPO)单元的几个重要作用。增强的机械理解将使设计新颖的对映选择性C–H转换成为可能。
更新日期:2020-11-13
中文翻译:

了解在镍催化的咪唑对映选择性C–H环化反应中,镍/ JoSPOphos歧管的独特反应模式
3d过渡金属催化的对映选择性C–H官能化为构建手性分子提供了可持续的策略。更好地理解反应的催化性质和控制对映选择性的因素对于合理设计更有效的系统非常重要。在本文中,通过密度泛函理论(DFT)计算研究了Ni催化的咪唑对映体C–H环化的机理。π-烯丙基镍(II)促进的σ络合物辅助复分解(σ-CAM)和镍(0)催化的氧化加成(OA)机理都是不利的。除了通常提出的配体到配体氢转移(LLHT)机理外,反应还可以通过一种非常规的σ-CAM机理,涉及氢从JoSPOphos配体通过P–H氧化加成/迁移插入,通过σ-CAM活化C(sp 2)–H以及C–C还原消除的氢转移。重要的是,基于这种新机制的计算结果确实可以重现实验观察到的对映选择性。此外,π烯丙基镍(催化活性II)配合物可以通过将活性镍(0)催化剂的再生合理化经由逐步的氢转移,已通过实验研究证实。该计算揭示了在反应过程中,JoSPOphos中次膦氧化物(SPO)单元的几个重要作用。增强的机械理解将使设计新颖的对映选择性C–H转换成为可能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号