当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insights of selective syngas conversion over Zn grafted on ZSM-5 zeolite
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0cy01739f Wei Chen 1, 2, 3, 4, 5 , Dinesh Acharya 1, 2, 3, 4, 5 , Zhiqiang Liu 1, 2, 3, 4, 5 , Xianfeng Yi 1, 2, 3, 4, 5 , Yao Xiao 1, 2, 3, 4, 5 , Xiaomin Tang 1, 2, 3, 4, 5 , Wenli Peng 1, 2, 3, 4, 5 , Anmin Zheng 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-02 , DOI: 10.1039/d0cy01739f Wei Chen 1, 2, 3, 4, 5 , Dinesh Acharya 1, 2, 3, 4, 5 , Zhiqiang Liu 1, 2, 3, 4, 5 , Xianfeng Yi 1, 2, 3, 4, 5 , Yao Xiao 1, 2, 3, 4, 5 , Xiaomin Tang 1, 2, 3, 4, 5 , Wenli Peng 1, 2, 3, 4, 5 , Anmin Zheng 1, 2, 3, 4, 5
Affiliation
|
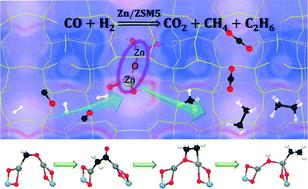
Metal-exchanged zeolites have attracted broad interest in alkane activation, NH3 selective catalytic reduction (NH3-SCR) reactions etc. Very recently, Chen et al. (Angew. Chem. Int. Ed. 2020, 59, 6529–6534) reported that Zn2+-ion exchanged aluminosilicate zeolites catalyze syngas conversion efficiently toward ethane with a high selectivity. Although the main active site of the catalyst has been proposed to be [Zn–O–Zn]2+ by solid state NMR experiments, the detailed reaction mechanism remains to be elucidated. Based on the intermediates detected experimentally, theoretical calculations herein propose a reaction pathway for ethane formation along with the intermediates [Zn–OCH2–Zn]2+ → [Zn–CH2CO–Zn]2+ → [Zn–OCH2CH2–Zn]2+ → [Zn–OH–Zn–C2H5]2+ grafted on a ZSM-5 zeolite. Combined with the kinetic analysis of the reaction pathways and the Gibbs free energy surface of alkane formation, the overall syngas conversion to ethane is nicely consistent with the experimental observations. On the basis of the evolution of the natural orbitals along the reaction coordinates, ZSM-5 not only provides an environment on which to graft the highly dispersed Zn species as [Zn–O–Zn]2+ but also decreases the barriers by efficient charge transfer. The in-depth understanding gained in this work may provide important guidance for the more rational design and optimization of catalysts for syngas conversion.
中文翻译:

ZSM-5沸石上锌选择性合成气转化的机理研究
金属交换的沸石,吸引在烷烃活化广泛兴趣,NH 3选择性催化还原(NH 3 -SCR)反应等最近,陈等人。(Angew。化学式中间体版2020,59,6529-6534)报道的Zn 2+ -离子以高选择性有效地交换的硅铝酸盐沸石催化合成气转化朝向乙烷。尽管已提出催化剂的主要活性部位为[Zn–O–Zn] 2+通过固态NMR实验,详细的反应机理还有待阐明。基于实验检测到的中间体,本文的理论计算提出了乙烷形成的反应途径以及中间体[Zn–OCH 2 –Zn] 2 + →[Zn–CH 2 CO–Zn] 2 + →[Zn–OCH 2 CH 2 –Zn] 2 + →[Zn–OH–Zn–C 2 H 5 ] 2+接枝在ZSM-5沸石上。结合反应路径的动力学分析和烷烃形成的吉布斯自由能面,合成气向乙烷的总转化率与实验观察结果非常吻合。根据自然轨道沿反应坐标的演变,ZSM-5不仅提供了一个环境,使高度分散的锌物种以[Zn–O–Zn] 2+的形式接枝,而且通过有效电荷降低了势垒转让。在这项工作中获得的深入理解可能为合成气转化催化剂的更合理设计和优化提供重要指导。
更新日期:2020-11-13
中文翻译:

ZSM-5沸石上锌选择性合成气转化的机理研究
金属交换的沸石,吸引在烷烃活化广泛兴趣,NH 3选择性催化还原(NH 3 -SCR)反应等最近,陈等人。(Angew。化学式中间体版2020,59,6529-6534)报道的Zn 2+ -离子以高选择性有效地交换的硅铝酸盐沸石催化合成气转化朝向乙烷。尽管已提出催化剂的主要活性部位为[Zn–O–Zn] 2+通过固态NMR实验,详细的反应机理还有待阐明。基于实验检测到的中间体,本文的理论计算提出了乙烷形成的反应途径以及中间体[Zn–OCH 2 –Zn] 2 + →[Zn–CH 2 CO–Zn] 2 + →[Zn–OCH 2 CH 2 –Zn] 2 + →[Zn–OH–Zn–C 2 H 5 ] 2+接枝在ZSM-5沸石上。结合反应路径的动力学分析和烷烃形成的吉布斯自由能面,合成气向乙烷的总转化率与实验观察结果非常吻合。根据自然轨道沿反应坐标的演变,ZSM-5不仅提供了一个环境,使高度分散的锌物种以[Zn–O–Zn] 2+的形式接枝,而且通过有效电荷降低了势垒转让。在这项工作中获得的深入理解可能为合成气转化催化剂的更合理设计和优化提供重要指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号