当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heteroatom-Doped Transition Metal Nitrides for CO Electrochemical Reduction: A Density Functional Theory Screening Study
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcc.0c08832 Mohammadreza Karamad 1 , Amir Barati Farimani 2 , Rishikesh Magar 2 , Samira Siahrostami 3 , Ian D. Gates 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcc.0c08832 Mohammadreza Karamad 1 , Amir Barati Farimani 2 , Rishikesh Magar 2 , Samira Siahrostami 3 , Ian D. Gates 1
Affiliation
|
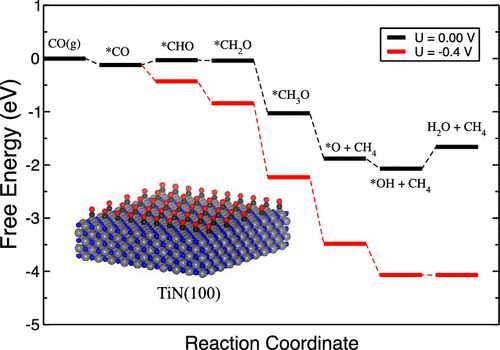
Electrochemical reduction of CO2 to fuels has attracted a great deal of attention in recent years as a potential solution to close the carbon cycle. In search for novel catalyst materials, we present in this study density functional theory-based screening of heteroatom-doped transition metal nitrides. We first examine zinc blend and rock salt polymorphs of transition metal nitrides and then consider doping the surface with 25% B, P, Sb, Bi, and C heteroatoms. We fully assess the stability and activity of the examined catalysts. The catalytic activity is measured by the calculated limiting potential, and the stability is assessed against hydroxyl (*OH) poisoning as well as dissolution under CO reduction relevant potentials. Of the screened nitrides, many are predicted to be active for the CO reduction reaction but only a few are stable under electrochemical conditions. In particular, on nearly all of the metal nitrides with a desired catalytic activity for CO reduction, either the competing hydrogen evolution reaction prevails over CO reduction or *OH poisoning occurs at CO reduction onset potentials. We identify five promising candidates including P-doped NbN, C-doped VN, P-doped VN, TiN, and Sb-doped TiN. Ultimately, this study emphasizes the relevance of stability under electrochemical conditions and the importance of taking into account the competition between the CO reduction, hydrogen evolution, and *OH reduction reactions under electrochemical conditions.
中文翻译:

用于CO电化学还原的杂原子掺杂过渡金属氮化物:密度泛函理论的筛选研究
电化学还原CO 2作为关闭碳循环的潜在解决方案,近年来燃料的使用引起了广泛关注。为了寻找新型催化剂材料,我们在本研究中介绍了基于密度泛函理论的杂原子掺杂过渡金属氮化物的筛选。我们首先检查过渡金属氮化物的锌共混物和岩盐多晶型物,然后考虑用25%的B,P,Sb,Bi和C杂原子掺杂表面。我们充分评估了所检查催化剂的稳定性和活性。通过计算的极限电势来测量催化活性,并评估其对羟基(* OH)中毒以及在CO还原相关电势下的溶解性的稳定性。在筛选出的氮化物中,预计许多对CO还原反应具有活性,但只有少数在电化学条件下稳定。特别是,在几乎所有对CO还原具有所需催化活性的金属氮化物上,竞争性的析氢反应胜过CO还原,或者* OH中毒发生在CO还原起始电位上。我们确定了五个有前途的候选人,包括P掺杂NbN,C掺杂VN,P掺杂VN,TiN和Sb掺杂TiN。最终,这项研究强调了在电化学条件下稳定性的相关性,以及在电化学条件下考虑到CO还原,氢释放和* OH还原反应之间竞争的重要性。我们确定了五个有前途的候选人,包括P掺杂NbN,C掺杂VN,P掺杂VN,TiN和Sb掺杂TiN。最终,这项研究强调了在电化学条件下稳定性的相关性,以及在电化学条件下考虑到CO还原,氢释放和* OH还原反应之间竞争的重要性。我们确定了五个有前途的候选人,包括P掺杂NbN,C掺杂VN,P掺杂VN,TiN和Sb掺杂TiN。最终,这项研究强调了在电化学条件下稳定性的相关性,以及在电化学条件下考虑到CO还原,氢释放和* OH还原反应之间竞争的重要性。
更新日期:2020-12-03
中文翻译:

用于CO电化学还原的杂原子掺杂过渡金属氮化物:密度泛函理论的筛选研究
电化学还原CO 2作为关闭碳循环的潜在解决方案,近年来燃料的使用引起了广泛关注。为了寻找新型催化剂材料,我们在本研究中介绍了基于密度泛函理论的杂原子掺杂过渡金属氮化物的筛选。我们首先检查过渡金属氮化物的锌共混物和岩盐多晶型物,然后考虑用25%的B,P,Sb,Bi和C杂原子掺杂表面。我们充分评估了所检查催化剂的稳定性和活性。通过计算的极限电势来测量催化活性,并评估其对羟基(* OH)中毒以及在CO还原相关电势下的溶解性的稳定性。在筛选出的氮化物中,预计许多对CO还原反应具有活性,但只有少数在电化学条件下稳定。特别是,在几乎所有对CO还原具有所需催化活性的金属氮化物上,竞争性的析氢反应胜过CO还原,或者* OH中毒发生在CO还原起始电位上。我们确定了五个有前途的候选人,包括P掺杂NbN,C掺杂VN,P掺杂VN,TiN和Sb掺杂TiN。最终,这项研究强调了在电化学条件下稳定性的相关性,以及在电化学条件下考虑到CO还原,氢释放和* OH还原反应之间竞争的重要性。我们确定了五个有前途的候选人,包括P掺杂NbN,C掺杂VN,P掺杂VN,TiN和Sb掺杂TiN。最终,这项研究强调了在电化学条件下稳定性的相关性,以及在电化学条件下考虑到CO还原,氢释放和* OH还原反应之间竞争的重要性。我们确定了五个有前途的候选人,包括P掺杂NbN,C掺杂VN,P掺杂VN,TiN和Sb掺杂TiN。最终,这项研究强调了在电化学条件下稳定性的相关性,以及在电化学条件下考虑到CO还原,氢释放和* OH还原反应之间竞争的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号