当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced Intermolecular Electron Transfer in Gas Phase Ion Pairs of the 1-Ethyl-3-methylimidazolium Cation and the Bis(trifluoromethylsulfonyl)imide Anion
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-13 , DOI: 10.1021/acs.jpca.0c06018 Ryan S. Booth 1 , Christopher J. Annesley 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-13 , DOI: 10.1021/acs.jpca.0c06018 Ryan S. Booth 1 , Christopher J. Annesley 2
Affiliation
|
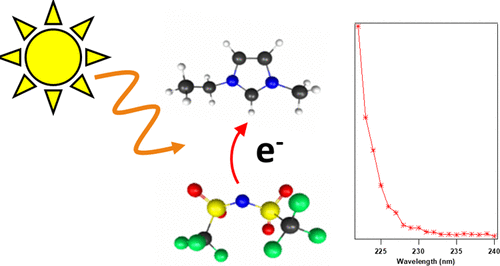
In this study, the UV photodissociation of gas phase ion pairs of the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, [emim]+[tf2n]−, is shown to proceed primarily through radical intermediates. [emim]+[tf2n]− ion pairs have been shown previously to undergo two-photon-dependent dissociation, but the mechanisms of this have not been probed in detail. By employing a two-laser pump probe spectroscopy and time-dependent density functional theory (TD-DFT) calculations, we have illustrated that one of the major UV photodissociation pathways in [emim]+[tf2n]− ion pairs is an intermolecular electron transfer wherein the anion transfers an electron to the cation resulting in two neutral open-shelled products. These products were observable for at least 1.6 μs post photodissociation, the experimental limit, via detection of the [emim]+ cation. This data demonstrates that the likely photoproducts of [emim]+[tf2n]− UV photodissociation are two neutral species that separate spatially, demonstrated through lack of observed relaxation pathways such as electron recombination. TD-DFT and frontier molecular orbital analysis calculations at the MN15/6-311++G(d,p) level are employed to aid in identifying excited state characteristics and support the interpretations of the experimental data. The energetic onset of the intermolecular electron transfer is consistent with previously observed [emim]+[tf2n]− absorption spectra in the bulk and gas phases. The similarities between bulk and gas phase UV spectra imply that this electron-transfer pathway may be a major photodissociation channel in both phases.
中文翻译:

1-乙基-3-甲基咪唑鎓阳离子和双(三氟甲基磺酰基)酰亚胺阴离子在气相离子对中的光诱导分子间电子转移
在这项研究中,离子液体1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)酰亚胺[emim] + [tf2n] -的气相离子对的UV光离解主要通过自由基中间体进行。先前已显示[emim] + [tf2n] -离子对经历了依赖于两个光子的解离,但是尚未详细探讨其机理。通过使用两激光泵浦光谱法和时变密度泛函理论(TD-DFT)计算,我们已经说明了[emim] + [tf2n]中的主要紫外线光解途径之一-离子对是一种分子间电子转移,其中阴离子将电子转移到阳离子上,产生两个中性的开壳产物。通过检测[emim] +阳离子,这些产物在光解后至少1.6μs即可观察到实验极限。该数据表明[emim] + [tf2n]的可能光产物-紫外线光解离是两个中性物种,它们在空间上分开,这是由于缺乏观察到的弛豫途径(如电子重组)所证实的。TD-DFT和MN15 / 6-311 ++ G(d,p)级的前沿分子轨道分析计算被用来帮助识别激发态特征并支持对实验数据的解释。分子间电子转移的能量开始与先前在本体相和气相中观察到的[emim] + [tf2n] -吸收光谱一致。本体和气相UV光谱之间的相似性暗示该电子传递途径可能是两个相中的主要光解离通道。
更新日期:2020-11-25
中文翻译:

1-乙基-3-甲基咪唑鎓阳离子和双(三氟甲基磺酰基)酰亚胺阴离子在气相离子对中的光诱导分子间电子转移
在这项研究中,离子液体1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)酰亚胺[emim] + [tf2n] -的气相离子对的UV光离解主要通过自由基中间体进行。先前已显示[emim] + [tf2n] -离子对经历了依赖于两个光子的解离,但是尚未详细探讨其机理。通过使用两激光泵浦光谱法和时变密度泛函理论(TD-DFT)计算,我们已经说明了[emim] + [tf2n]中的主要紫外线光解途径之一-离子对是一种分子间电子转移,其中阴离子将电子转移到阳离子上,产生两个中性的开壳产物。通过检测[emim] +阳离子,这些产物在光解后至少1.6μs即可观察到实验极限。该数据表明[emim] + [tf2n]的可能光产物-紫外线光解离是两个中性物种,它们在空间上分开,这是由于缺乏观察到的弛豫途径(如电子重组)所证实的。TD-DFT和MN15 / 6-311 ++ G(d,p)级的前沿分子轨道分析计算被用来帮助识别激发态特征并支持对实验数据的解释。分子间电子转移的能量开始与先前在本体相和气相中观察到的[emim] + [tf2n] -吸收光谱一致。本体和气相UV光谱之间的相似性暗示该电子传递途径可能是两个相中的主要光解离通道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号