当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Amino Acid Size on the Internal Dynamics and Conformational Freedom of Polypeptides
Macromolecules ( IF 5.1 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.macromol.0c02153 Remi Casier 1 , Jean Duhamel 1
Macromolecules ( IF 5.1 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.macromol.0c02153 Remi Casier 1 , Jean Duhamel 1
Affiliation
|
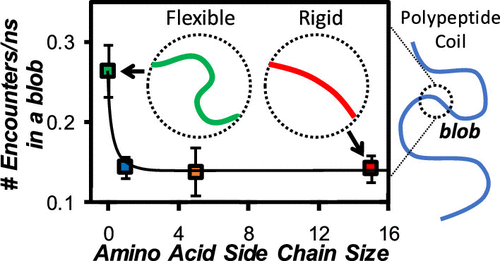
The fluorescence blob model (FBM) was applied to analyze the fluorescence decays of a series of pyrene-labeled polypeptides to better understand how the amino acid (aa) composition of a polypeptide affected its dynamics. Three pyrene-labeled polypeptides were prepared by copolymerizing racemic (d,l) mixtures of different aa’s, namely, glycine (Gly), alanine (Ala), and carbobenzyloxylysine (Lys(Z)) with glutamic acid (Glu) to yield Py-PGlyGlu, Py-PAlaGlu, and Py-PLys(Z)Glu, respectively. All polypeptides contained 44 (± 3) mol % Glu for fluorescence labeling with 1-pyrenemethylamine. The behavior of these three polypeptides was characterized in N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) by fluorescence. It was then compared with the behavior of pyrene-labeled poly(d,l-glutamic acid) (Py-PGlu) to assess the effect that each comonomer had on the backbone dynamics of the polypeptides. The FBM analysis of the fluorescence decays yielded the maximum number (Nblob) of residues separating two Glu’s bearing a pyrenyl group while still allowing excimer formation between an excited and a ground-state pyrene. Py-PLys(Z)Glu yielded the same blob size (Nblob = 11) as for the Py-PGlu samples. In contrast, the incorporation of ∼56 mol % Ala and Gly resulted in an increase in Nblob from 11 for Py-PGlu and Py-PLys(Z)Glu to 16 for Py-PAlaGlu and 23 for Py-PGlyGlu in DMSO. Considering that the internal dynamics of a polymer depend strongly on the size of its structural units and keeping proline aside, whose cyclic structure prevents backbone motion, this result implied that Nblob for the 17 largest aa’s of the 20 most common aa’s with two or more atoms in their side chain must take a value between 11 for PGlu and 16 for PAlaGlu. This rather narrow range of Nblob values suggests that the internal dynamics of polypeptides should be much simpler to predict than their structure since only two aa’s, namely, Gly and Ala, out of the 20 most common aa’s, appear to contribute differently to the internal dynamics of polypeptides.
中文翻译:

氨基酸大小对多肽内部动力学和构象自由度的影响
荧光斑点模型(FBM)用于分析一系列of标记的多肽的荧光衰减,以更好地了解多肽的氨基酸(aa)组成如何影响其动力学。通过将不同氨基酸的外消旋混合物(d,l)与甘氨酸(Glu)共聚生成甘氨酸(Gly),丙氨酸(Ala)和羧苄氧基赖氨酸(Lys(Z))的三种pyr标记的多肽Py-PGlyGlu,Py-PAlaGlu和Py-PLys(Z)Glu。所有多肽均包含44(±3)mol%Glu,用于用1-py甲基胺进行荧光标记。这三种多肽的行为以N,N为特征-二甲基甲酰胺(DMF)和二甲基亚砜(DMSO)的荧光作用。然后将其与of标记的聚(d,1-谷氨酸)(Py-PGlu)的行为进行比较,以评估每种共聚单体对多肽的骨架动力学的影响。对荧光衰减进行的FBM分析得出了最大数目的残基(N blob),该残基将两个带有a基的Glu分开,同时仍允许在激发态和基态between之间形成受激准分子。Py-PLys(Z)Glu产生的斑点大小与Py-PGlu样品相同(N斑点= 11)。相反,掺入〜56 mol%的Ala和Gly导致N斑点增加从DMSO中的Py-PGlu和Py-PLys(Z)Glu为11到Py-PAlaGlu为16和Py-PGlyGlu为23。考虑到聚合物的内部动力学上的其结构单元和保持脯氨酸尺寸强烈依赖一边,其环状结构防止骨干运动,该结果暗示,Ñ团块为17最大AA “S中的20个最常见的氨基酸的在其侧链中具有两个或多个原子的原子,其PGlu值必须为11,而PAlaGlu值必须为16。N斑点值的范围相当狭窄,表明多肽的内部动力学比其结构更容易预测,因为在20种最常见的氨基酸中只有两个氨基酸,即Gly和Ala似乎对多肽的内部动力学有不同的贡献。
更新日期:2020-11-25
中文翻译:

氨基酸大小对多肽内部动力学和构象自由度的影响
荧光斑点模型(FBM)用于分析一系列of标记的多肽的荧光衰减,以更好地了解多肽的氨基酸(aa)组成如何影响其动力学。通过将不同氨基酸的外消旋混合物(d,l)与甘氨酸(Glu)共聚生成甘氨酸(Gly),丙氨酸(Ala)和羧苄氧基赖氨酸(Lys(Z))的三种pyr标记的多肽Py-PGlyGlu,Py-PAlaGlu和Py-PLys(Z)Glu。所有多肽均包含44(±3)mol%Glu,用于用1-py甲基胺进行荧光标记。这三种多肽的行为以N,N为特征-二甲基甲酰胺(DMF)和二甲基亚砜(DMSO)的荧光作用。然后将其与of标记的聚(d,1-谷氨酸)(Py-PGlu)的行为进行比较,以评估每种共聚单体对多肽的骨架动力学的影响。对荧光衰减进行的FBM分析得出了最大数目的残基(N blob),该残基将两个带有a基的Glu分开,同时仍允许在激发态和基态between之间形成受激准分子。Py-PLys(Z)Glu产生的斑点大小与Py-PGlu样品相同(N斑点= 11)。相反,掺入〜56 mol%的Ala和Gly导致N斑点增加从DMSO中的Py-PGlu和Py-PLys(Z)Glu为11到Py-PAlaGlu为16和Py-PGlyGlu为23。考虑到聚合物的内部动力学上的其结构单元和保持脯氨酸尺寸强烈依赖一边,其环状结构防止骨干运动,该结果暗示,Ñ团块为17最大AA “S中的20个最常见的氨基酸的在其侧链中具有两个或多个原子的原子,其PGlu值必须为11,而PAlaGlu值必须为16。N斑点值的范围相当狭窄,表明多肽的内部动力学比其结构更容易预测,因为在20种最常见的氨基酸中只有两个氨基酸,即Gly和Ala似乎对多肽的内部动力学有不同的贡献。










































 京公网安备 11010802027423号
京公网安备 11010802027423号