当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal Structure and Mechanistic Molecular Modeling Studies of Mycobacterium tuberculosis Diterpene Cyclase Rv3377c
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.biochem.0c00762 Yue Zhang 1 , Lisa M. Prach 2 , Terrence E. O’Brien 1 , Frank DiMaio 3 , Daniil M. Prigozhin 4 , Jacob E. Corn 5 , Tom Alber 6 , Justin B. Siegel 1, 7, 8 , Dean J. Tantillo 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.biochem.0c00762 Yue Zhang 1 , Lisa M. Prach 2 , Terrence E. O’Brien 1 , Frank DiMaio 3 , Daniil M. Prigozhin 4 , Jacob E. Corn 5 , Tom Alber 6 , Justin B. Siegel 1, 7, 8 , Dean J. Tantillo 1
Affiliation
|
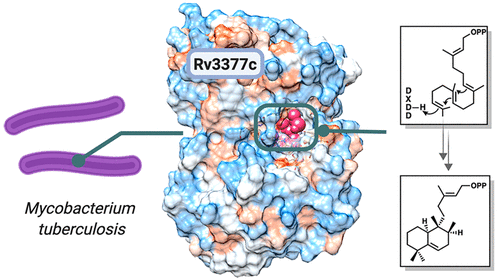
Terpenes make up the largest class of natural products, with extensive chemical and structural diversity. Diterpenes, mostly isolated from plants and rarely prokaryotes, exhibit a variety of important biological activities and valuable applications, including providing antitumor and antibiotic pharmaceuticals. These natural products are constructed by terpene synthases, a class of enzymes that catalyze one of the most complex chemical reactions in biology: converting simple acyclic oligo-isoprenyl diphosphate substrates to complex polycyclic products via carbocation intermediates. Here we obtained the second ever crystal structure of a class II diterpene synthase from bacteria, tuberculosinol pyrophosphate synthase (i.e., Halimadienyl diphosphate synthase, MtHPS, or Rv3377c) from Mycobacterium tuberculosis (Mtb). This enzyme transforms (E,E,E)-geranylgeranyl diphosphate into tuberculosinol pyrophosphate (Halimadienyl diphosphate). Rv3377c is part of the Mtb diterpene pathway along with Rv3378c, which converts tuberculosinol pyrophosphate to 1-tuberculosinyl adenosine (1-TbAd). This pathway was shown to exist only in virulent Mycobacterium species, but not in closely related avirulent species, and was proposed to be involved in phagolysosome maturation arrest. To gain further insight into the reaction pathway and the mechanistically relevant enzyme substrate binding orientation, electronic structure calculation and docking studies of reaction intermediates were carried out. Results reveal a plausible binding mode of the substrate that can provide the information to guide future drug design and anti-infective therapies of this biosynthetic pathway.
中文翻译:

结核分枝杆菌二萜环化酶Rv3377c的晶体结构和分子机理研究
萜烯是最大的天然产物类别,具有广泛的化学和结构多样性。二萜主要从植物中分离而很少原核生物,它们具有多种重要的生物学活性和有价值的应用,包括提供抗肿瘤和抗生素药物。这些天然产物是由萜烯合酶构建的,萜烯合酶是催化生物学上最复杂的化学反应之一的酶:通过碳正离子中间体将简单的无环低聚异戊二烯基二磷酸底物转化为复杂的多环产物。在这里,我们从细菌结核分枝杆菌(MTB)。该酶将(E,E,E)-香叶基香叶基二磷酸转化为结核菌醇焦磷酸酯(Halimadienyl diphosphate)。Rv3377c与Rv3378c一起是Mtb二萜途径的一部分,Rv3378c可以将焦磷酸焦磷酸烷基酚转化为1-结核氨酰基腺苷(1-TbAd)。已证明该途径仅存在于强力分枝杆菌中物种,但没有密切相关的无毒物种,并被提议参与吞噬溶酶体的成熟停滞。为了进一步了解反应途径和与机械相关的酶底物结合方向,进行了电子结构计算和反应中间体的对接研究。结果揭示了底物的合理结合模式,该模式可以提供信息以指导该生物合成途径的未来药物设计和抗感染治疗。
更新日期:2020-12-01
中文翻译:

结核分枝杆菌二萜环化酶Rv3377c的晶体结构和分子机理研究
萜烯是最大的天然产物类别,具有广泛的化学和结构多样性。二萜主要从植物中分离而很少原核生物,它们具有多种重要的生物学活性和有价值的应用,包括提供抗肿瘤和抗生素药物。这些天然产物是由萜烯合酶构建的,萜烯合酶是催化生物学上最复杂的化学反应之一的酶:通过碳正离子中间体将简单的无环低聚异戊二烯基二磷酸底物转化为复杂的多环产物。在这里,我们从细菌结核分枝杆菌(MTB)。该酶将(E,E,E)-香叶基香叶基二磷酸转化为结核菌醇焦磷酸酯(Halimadienyl diphosphate)。Rv3377c与Rv3378c一起是Mtb二萜途径的一部分,Rv3378c可以将焦磷酸焦磷酸烷基酚转化为1-结核氨酰基腺苷(1-TbAd)。已证明该途径仅存在于强力分枝杆菌中物种,但没有密切相关的无毒物种,并被提议参与吞噬溶酶体的成熟停滞。为了进一步了解反应途径和与机械相关的酶底物结合方向,进行了电子结构计算和反应中间体的对接研究。结果揭示了底物的合理结合模式,该模式可以提供信息以指导该生物合成途径的未来药物设计和抗感染治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号