当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyphenol Honokiol and Flavone 2′,3′,4′-Trihydroxyflavone Differentially Interact with α-Synuclein at Distinct Phases of Aggregation
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-11-13 , DOI: 10.1021/acschemneuro.0c00654 Blagojce Jovcevski 1, 2, 3 , Sukanya Das 4 , Scott Smid 5 , Tara Louise Pukala 1, 3
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-11-13 , DOI: 10.1021/acschemneuro.0c00654 Blagojce Jovcevski 1, 2, 3 , Sukanya Das 4 , Scott Smid 5 , Tara Louise Pukala 1, 3
Affiliation
|
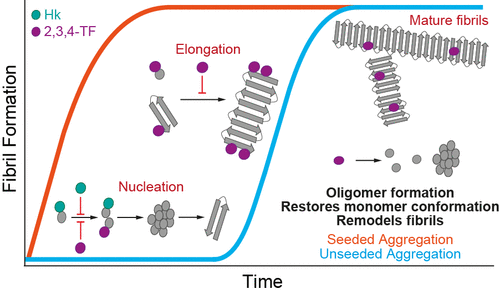
The association between protein aggregation and neurodegenerative diseases such as Parkinson’s disease continues to be well interrogated but poorly elucidated at a mechanistic level. Nevertheless, the formation of amyloid fibrils from the destabilization and misfolding of native proteins is a molecular hallmark of disease. Consequently, there is ongoing demand for the identification and development of small molecules which prevent fibril formation. This study comprehensively assesses the inhibitory properties of two small molecules, the lignan polyphenol honokiol and the flavonoid 2′,3′,4′-trihydroxyflavone, in preventing α-synuclein fibrilization. The data shows that honokiol does not prevent α-synuclein fibril elongation, while 2′,3′,4′-trihydroxyflavone is effective at inhibiting fibril elongation and induces oligomer formation (for both wild-type α-synuclein and the disease-associated A53T mutation). Moreover, the exposed hydrophobicity of α-synuclein fibrils is reduced in the presence of 2′,3′,4′-trihydroxyflavone, whereas the addition of honokiol did not reduce the hydrophobicity of fibrils. In addition, ion mobility–mass spectrometry revealed that the conformation of α-synuclein wild-type and A53T monomers after disassembly is restored to a nonaggregation-prone state upon 2′,3′,4′-trihydroxyflavone treatment. Collectively, this study shows that the mechanisms by which these polyphenols and flavonoids prevent fibril formation are distinct by their interactions at various phases of the fibril-forming pathway. Furthermore, this study highlights how thorough biophysical interrogation of the interaction is required for understanding the ability of inhibitors to prevent protein aggregation associated with disease.
中文翻译:

多酚厚朴酚和黄酮2',3',4'-三羟基黄酮在聚集的不同阶段与α-突触核蛋白差异相互作用
蛋白质聚集与神经退行性疾病(如帕金森氏病)之间的关联仍然受到很好的质疑,但在机械水平上却难以阐明。然而,由于天然蛋白的不稳定和错误折叠而形成淀粉样蛋白原纤维是疾病的分子标志。因此,持续需要鉴定和开发防止原纤维形成的小分子。这项研究全面评估了两个小分子木脂素多酚厚朴酚和类黄酮2',3',4'-三羟基黄酮在预防α-突触核蛋白纤维化中的抑制作用。数据显示,厚朴酚并不能阻止α-突触核蛋白原纤维的伸长,而2',3' 4'-三羟基黄酮有效抑制原纤维伸长并诱导寡聚物形成(对于野生型α-突触核蛋白和与疾病相关的A53T突变)。此外,在2',3',4'-三羟基黄酮的存在下,α-突触核蛋白原纤维的暴露疏水性降低,而厚朴酚的添加并未降低原纤维的疏水性。此外,离子淌度质谱分析表明,经2',3',4'-三羟基黄酮处理后,分解后的α-突触核蛋白野生型和A53T单体构象恢复为非聚集态。总的来说,这项研究表明,这些多酚和类黄酮防止原纤维形成的机制在原纤维形成途径的各个阶段的相互作用是不同的。此外,
更新日期:2020-12-16
中文翻译:

多酚厚朴酚和黄酮2',3',4'-三羟基黄酮在聚集的不同阶段与α-突触核蛋白差异相互作用
蛋白质聚集与神经退行性疾病(如帕金森氏病)之间的关联仍然受到很好的质疑,但在机械水平上却难以阐明。然而,由于天然蛋白的不稳定和错误折叠而形成淀粉样蛋白原纤维是疾病的分子标志。因此,持续需要鉴定和开发防止原纤维形成的小分子。这项研究全面评估了两个小分子木脂素多酚厚朴酚和类黄酮2',3',4'-三羟基黄酮在预防α-突触核蛋白纤维化中的抑制作用。数据显示,厚朴酚并不能阻止α-突触核蛋白原纤维的伸长,而2',3' 4'-三羟基黄酮有效抑制原纤维伸长并诱导寡聚物形成(对于野生型α-突触核蛋白和与疾病相关的A53T突变)。此外,在2',3',4'-三羟基黄酮的存在下,α-突触核蛋白原纤维的暴露疏水性降低,而厚朴酚的添加并未降低原纤维的疏水性。此外,离子淌度质谱分析表明,经2',3',4'-三羟基黄酮处理后,分解后的α-突触核蛋白野生型和A53T单体构象恢复为非聚集态。总的来说,这项研究表明,这些多酚和类黄酮防止原纤维形成的机制在原纤维形成途径的各个阶段的相互作用是不同的。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号