Synthesis ( IF 2.2 ) Pub Date : 2020-11-12 , DOI: 10.1055/s-0040-1705956 Peng Lei 1, 2, 3 , Guangchen Li 3 , Michal Szostak 3 , Yun Ling 2 , Jie An 4 , Steven P. Nolan 5
|
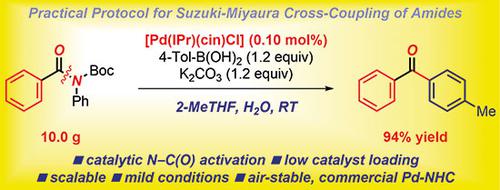
Amides are among the most important and ubiquitous functional groups in organic chemistry and process development. In this Practical Synthetic Procedure, a protocol for the Suzuki–Miyaura cross-coupling of amides by selective N–C(O) bond activation catalyzed by commercially available, air- and moisture-stable palladium/N-heterocyclic carbene (NHC) complexes is described. The procedure described involves [Pd(IPr)(cin)Cl] [IPr = 2,6-(diisopropylphenyl)imidazol-2-ylidene, cin = cinnamyl] at 0.10 mol% at room temperature and is performed on decagram scale. Furthermore, a procedure for the synthesis of amide starting materials is accomplished via selective N-tert-butoxycarbonylation, which is the preferred method over N-acylation. The present protocol carries advantages of operational simplicity, commercial availability of catalysts, and excellent conversions at low catalyst loadings. The method is generally useful for activation of N–C(O) amide bonds in a broad spectrum of amide precursors. The protocol should facilitate the implementation of amide cross-coupling reactions.
中文翻译:

钯/ N-杂环卡宾催化的NC-(O)活化的铃木-宫浦酰胺交叉偶联反应方案
酰胺是有机化学和工艺开发中最重要和最普遍的官能团之一。在此实用的合成程序中,通过市售的空气和水分稳定的钯/ N-杂环卡宾(NHC)络合物催化的选择性N-C(O)键活化,使铃木-Miyaura酰胺交叉偶联的方案为描述。所描述的步骤涉及[Pd(IPr)(cin)Cl] [IPr = 2,6-(二异丙基苯基)咪唑-2-亚烷基,cin =肉桂基],室温下以0.10 mol%进行,并以十克级进行。此外,通过选择性N-叔胺完成了酰胺起始原料的合成过程。-丁氧基羰基化,这是优于N-酰化的优选方法。本方案具有操作简单,催化剂的商业可获得性和在低催化剂负载下的优异转化率的优点。该方法通常可用于在广泛的酰胺前体中活化N–C(O)酰胺键。该方案应促进酰胺交叉偶联反应的实施。











































 京公网安备 11010802027423号
京公网安备 11010802027423号