当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ubiquitination and the proteasome rather than caspase-3-mediated C-terminal cleavage are involved in the EAAT2 degradation by staurosporine-induced cellular stress
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-11-12 , DOI: 10.1111/jnc.15237 Timo-Daniel Voss 1 , Maria Gerget 1 , Birgit Linkus 1 , Bjoern von Einem 1 , G Bernhard Landwehrmeyer 1 , Jan Lewerenz 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-11-12 , DOI: 10.1111/jnc.15237 Timo-Daniel Voss 1 , Maria Gerget 1 , Birgit Linkus 1 , Bjoern von Einem 1 , G Bernhard Landwehrmeyer 1 , Jan Lewerenz 1
Affiliation
|
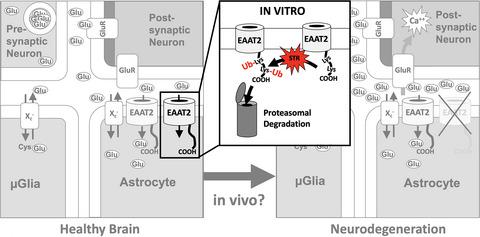
Diminished glutamate (Glu) uptake via the excitatory amino acid transporter EAAT2, which normally accounts for ~90% of total forebrain EAAT activity, may contribute to neurodegeneration via Glu-mediated excitotoxicity. C-terminal cleavage by caspase-3 (C3) was reported to mediate EAAT2 inactivation and down-regulation in the context of neurodegeneration. For a detailed analysis of C3-dependent EAAT2 degradation, we employed A172 glioblastoma as well as hippocampal HT22 cells and murine astrocytes over-expressing VSV-G-tagged EAAT2 constructs. C3 activation was induced by staurosporine (STR). In HT22 cells, STR-induced C3 activation-induced rapid EAAT2 protein degradation. The mutation of asparagine 504 to aspartate (D504N), which should inactivate the putative C3 cleavage site, increased EAAT2 activity in A172 cells. In contrast, the D504N mutation did not protect EAAT2 protein against STR-induced degradation in HT22 cells, whereas inhibition of caspases, ubiquitination and the proteasome did. Similar results were obtained in astrocytes. Phylogenetic analysis showed that C-terminal ubiquitin acceptor sites—but not the putative C3 cleavage site—exhibit a high degree of conservation. Moreover, C-terminal truncation mimicking C3 cleavage increased rather than decreased EAAT2 activity and stability as well as protected EAAT2 against STR-induced ubiquitination-dependent degradation. We conclude that cellular stress associated with endogenous C3 activation degrades EAAT2 via a pathway involving ubiquitination and the proteasome but not direct C3-mediated cleavage. In addition, C3 cleavage of EAAT2, described to occur in other models, is unlikely to inactivate EAAT2. However, mutation of the highly conserved D504 within the putative C3 cleavage site increases EAAT2 activity via an unknown mechanism.
中文翻译:

泛素化和蛋白酶体而不是胱天蛋白酶3介导的C末端裂解参与星形孢菌素诱导的细胞应激对EAAT2的降解。
通过兴奋性氨基酸转运蛋白EAAT2吸收的谷氨酸(Glu)减少(通常占前脑EAAT总活性的90%),可能通过Glu介导的兴奋性毒性而导致神经退行性变。据报道,在神经变性的情况下,caspase-3(C3)的C末端切割介导了EAAT2的失活和下调。对于C3依赖性EAAT2降解的详细分析,我们使用了A172胶质母细胞瘤以及海马HT22细胞和鼠星形胶质细胞,它们过表达VSV-G标签的EAAT2构建体。C3激活是由星形孢菌素(STR)诱导的。在HT22细胞中,STR诱导C3活化导致EAAT2蛋白快速降解。天冬酰胺504突变为天冬氨酸(D504N),该突变应使假定的C3切割位点失活,从而增加了A172细胞的EAAT2活性。相比之下,D504N突变不能保护EAAT2蛋白免受STR诱导的HT22细胞降解,而抑制半胱天冬酶,泛素化和蛋白酶体却可以。在星形胶质细胞中获得了相似的结果。系统发育分析表明,C末端泛素受体位点(而不是假定的C3裂解位点)表现出高度的保守性。此外,模拟C3裂解的C端截短增加而不是降低EAAT2的活性和稳定性,并保护EAAT2免受STR诱导的泛素依赖性降解。我们得出的结论是,与内源性C3激活相关的细胞应激通过涉及泛素化和蛋白酶体的途径降解EAAT2,但不直接介导C3介导的裂解。此外,据报道发生在其他模型中的EAAT2的C3裂解不太可能使EAAT2失活。然而,
更新日期:2020-11-12
中文翻译:

泛素化和蛋白酶体而不是胱天蛋白酶3介导的C末端裂解参与星形孢菌素诱导的细胞应激对EAAT2的降解。
通过兴奋性氨基酸转运蛋白EAAT2吸收的谷氨酸(Glu)减少(通常占前脑EAAT总活性的90%),可能通过Glu介导的兴奋性毒性而导致神经退行性变。据报道,在神经变性的情况下,caspase-3(C3)的C末端切割介导了EAAT2的失活和下调。对于C3依赖性EAAT2降解的详细分析,我们使用了A172胶质母细胞瘤以及海马HT22细胞和鼠星形胶质细胞,它们过表达VSV-G标签的EAAT2构建体。C3激活是由星形孢菌素(STR)诱导的。在HT22细胞中,STR诱导C3活化导致EAAT2蛋白快速降解。天冬酰胺504突变为天冬氨酸(D504N),该突变应使假定的C3切割位点失活,从而增加了A172细胞的EAAT2活性。相比之下,D504N突变不能保护EAAT2蛋白免受STR诱导的HT22细胞降解,而抑制半胱天冬酶,泛素化和蛋白酶体却可以。在星形胶质细胞中获得了相似的结果。系统发育分析表明,C末端泛素受体位点(而不是假定的C3裂解位点)表现出高度的保守性。此外,模拟C3裂解的C端截短增加而不是降低EAAT2的活性和稳定性,并保护EAAT2免受STR诱导的泛素依赖性降解。我们得出的结论是,与内源性C3激活相关的细胞应激通过涉及泛素化和蛋白酶体的途径降解EAAT2,但不直接介导C3介导的裂解。此外,据报道发生在其他模型中的EAAT2的C3裂解不太可能使EAAT2失活。然而,











































 京公网安备 11010802027423号
京公网安备 11010802027423号