Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-11-13 , DOI: 10.1016/j.jre.2020.11.008 Wencai Zhang 1 , Aaron Noble 1 , Bin Ji 1 , Qi Li 1
|
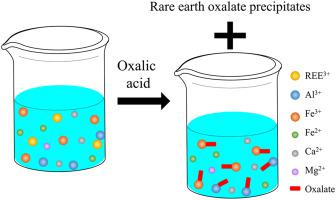
Solution equilibrium calculations were performed in this study to understand the impact of contaminant metal ions on the precipitation efficiency of selected rare earth elements (Ce3+, Nd3+, and Y3+) using oxalic acid as a precipitant. Trivalent metal ions, Al3+ and Fe3+, are found to considerably affect the precipitation efficiency of REEs. When Al3+ and Fe3+ concentrations are increased by 1 × 10−4 mol/L, in order to achieve an acceptable cerium recovery of 93% from solutions containing 1 × 10−4 mol/L Ce3+, oxalate dosage needs to increase by 1.2 × 10−4 and 1.68 × 10−4 mol/L, respectively. Such great impacts on the required oxalate dosage are also observed for Nd3+ and Y3+, which indicates that oxalic acid consumption and cost will be largely increased when the trivalent metal ions exist in REE-concentrated solutions. Effects of the divalent metal ions on the oxalate dosage are minimal. Furthermore, solution equilibrium calculation results show that the precipitation of Fe3+ and Ca2+ (e.g., hematite and Ca(C2O4)∙H2O(s)) likely occurs during the oxalate precipitation of REEs at relatively high pH (e.g., pH 2.5), which will reduce rare earth oxalate product purity. In addition to the metal ions, anionic species, especially , are also found to negatively affect the precipitation recovery of REEs. For example, when 0.1 mol/L occurs in a solution containing 1 × 10−4 mol/L Ce3+ and 4 × 10−4 mol/L oxalate, the pH needs to be elevated from 2.0 to 3.3 to achieve the acceptable recovery. Overall, findings from this study provide guidance for the obtainment of high-purity rare earth products from solutions containing a considerable amount of contaminant metal ions by means of oxalic acid precipitation.
中文翻译:

污染金属离子对草酸沉淀回收稀土元素的影响
本研究进行溶液平衡计算,以了解污染物金属离子对使用草酸作为沉淀剂的选定稀土元素(Ce 3+、Nd 3+和 Y 3+ )的沉淀效率的影响。发现三价金属离子Al 3+和Fe 3+显着影响稀土元素的沉淀效率。当 Al 3+和 Fe 3+浓度增加 1 × 10 -4 mol/L 时,为了从含有 1 × 10 -4 mol/L Ce 3+的溶液中获得可接受的 93% 的铈回收率,需要草酸盐用量增加 1.2 × 10 -4和1.68 × 10 -4 mol/L。Nd 3+和Y 3+也观察到对所需草酸盐剂量的巨大影响,这表明当三价金属离子存在于REE浓缩溶液中时,草酸的消耗和成本将大大增加。二价金属离子对草酸盐剂量的影响很小。此外,溶液平衡计算结果表明,Fe 3+和Ca 2+(例如赤铁矿和Ca(C 2 O 4 )∙H 2 O (s)) 可能发生在 REE 在较高 pH(例如,pH 2.5)下的草酸盐沉淀过程中,这将降低稀土草酸盐产品的纯度。除了金属离子,阴离子物质,尤其是, 也被发现对 REE 的沉淀回收产生负面影响。例如,当 0.1 mol/L发生在含有 1 × 10 -4 mol/L Ce 3+和 4 × 10 -4 mol/L 草酸盐的溶液中,需要将 pH 从 2.0 提高到 3.3 才能达到可接受的回收率。总体而言,这项研究的结果为通过草酸沉淀从含有大量污染金属离子的溶液中获得高纯度稀土产品提供了指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号