Cell Host & Microbe ( IF 20.6 ) Pub Date : 2020-11-13 , DOI: 10.1016/j.chom.2020.11.001 Maolin Lu 1 , Pradeep D Uchil 1 , Wenwei Li 1 , Desheng Zheng 1 , Daniel S Terry 2 , Jason Gorman 3 , Wei Shi 3 , Baoshan Zhang 3 , Tongqing Zhou 3 , Shilei Ding 4 , Romain Gasser 4 , Jérémie Prévost 4 , Guillaume Beaudoin-Bussières 4 , Sai Priya Anand 5 , Annemarie Laumaea 4 , Jonathan R Grover 1 , Lihong Liu 6 , David D Ho 6 , John R Mascola 3 , Andrés Finzi 5 , Peter D Kwong 3 , Scott C Blanchard 2 , Walther Mothes 1
|
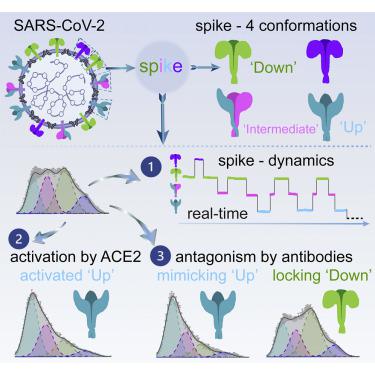
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) mediates viral entry into cells and is critical for vaccine development against coronavirus disease 2019 (COVID-19). Structural studies have revealed distinct conformations of S, but real-time information that connects these structures is lacking. Here we apply single-molecule fluorescence (Förster) resonance energy transfer (smFRET) imaging to observe conformational dynamics of S on virus particles. Virus-associated S dynamically samples at least four distinct conformational states. In response to human receptor angiotensin-converting enzyme 2 (hACE2), S opens sequentially into the hACE2-bound S conformation through at least one on-path intermediate. Conformational preferences observed upon exposure to convalescent plasma or antibodies suggest mechanisms of neutralization involving either competition with hACE2 for binding to the receptor-binding domain (RBD) or allosteric interference with conformational changes required for entry. Our findings inform on mechanisms of S recognition and conformations for immunogen design.
中文翻译:

病毒颗粒上 SARS-CoV-2 尖峰的实时构象动力学
严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 刺突 (S) 介导病毒进入细胞,对于开发针对 2019 年冠状病毒病 (COVID-19) 的疫苗至关重要。结构研究揭示了 S 的独特构象,但缺乏连接这些结构的实时信息。在这里,我们应用单分子荧光(Förster)共振能量转移(smFRET)成像来观察病毒颗粒上S的构象动力学。病毒相关的 S 动态采样至少四种不同的构象状态。响应人类受体血管紧张素转换酶 2 (hACE2),S 通过至少一种路径中间体顺序打开成 hACE2 结合的 S 构象。暴露于恢复期血浆或抗体时观察到的构象偏好表明中和机制涉及与 hACE2 竞争结合受体结合域 (RBD) 或对进入所需的构象变化进行变构干扰。我们的研究结果为免疫原设计的 S 识别机制和构象提供了信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号