Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-11-13 , DOI: 10.1016/j.bbamem.2020.183510 Ebehiremen N. Ayewoh , Lindsay C. Czuba , Thao T. Nguyen , Peter W. Swaan
|
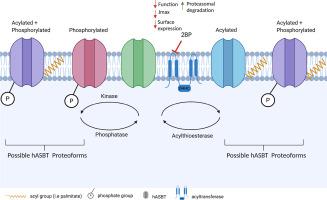
The human apical sodium-dependent bile acid transporter (hASBT, SLC10A2) is the rate-limiting step of intestinal bile acid absorption in the enterohepatic circulation system of bile acids. Therefore, the regulation and stability of hASBT is vital in maintaining bile acid and cholesterol homeostasis and may serve as a potential target for cholesterol-related disorders. We hypothesized that post-translational mechanisms that govern hASBT function and regulation will provide novel insight on intestinal bile acid transport and homeostasis. In this study, we confirm the S-acylation status of hASBT via acyl biotin exchange in COS-1 cells and its impact on hASBT expression, function, kinetics, and protein stability. Using the acylation inhibitor, 2-bromopalmitate, we show that S-acylation is an important modification which modulates the function, surface expression, and maximal transporter flux (Jmax) of hASBT. By means of proteasome inhibitors, S-acylated hASBT was found to be cleared via the proteasome whereas a reduction in the palmitoylation status of hASBT resulted in rapid proteolytic degradation compared to the unmodified transporter. Screening of cysteine mutants in and or near transmembrane domains, some of which are exposed to the cytosol, confirmed Cys314 to be the predominate S-acylated residue. Lastly, we show that S-acylation was reduced in a mutant form of hASBT devoid of cytosolic facing tyrosine residues, suggestive of crosstalk between acylation and phosphorylation post-translational modification mechanisms.
中文翻译:

胆汁酸转运蛋白hASBT的S-酰化状态调节其功能,代谢稳定性,膜表达和磷酸化状态
人根尖钠依赖性胆汁酸转运蛋白(hASBT,SLC10A2)是肠胆汁酸在胆汁酸肠肝循环系统中吸收的限速步骤。因此,hASBT的调节和稳定性对于维持胆汁酸和胆固醇的体内稳态至关重要,并且可能成为胆固醇相关疾病的潜在靶标。我们假设,控制hASBT功能和调控的翻译后机制将为肠道胆汁酸运输和体内平衡提供新的见解。在这项研究中,我们通过COS-1细胞中的酰基生物素交换确认了hASBT的S酰化状态及其对hASBT表达,功能,动力学和蛋白质稳定性的影响。使用酰化抑制剂2-溴棕榈酸酯,我们表明S-酰化是重要的修饰,其调节hASBT的功能,表面表达和最大转运蛋白通量(J max)。通过蛋白酶体抑制剂,发现S-酰化的hASBT可通过蛋白酶体清除,而与未修饰的转运蛋白相比,hASBT的棕榈酰化状态降低导致蛋白水解迅速降解。筛选跨膜结构域内或附近的半胱氨酸突变体,其中一些暴露于细胞质,证实Cys314是主要的S-酰化残基。最后,我们表明,小号-在没有细胞质面对酪氨酸残基的hASBT突变形式中,酰化作用降低,提示酰化作用与磷酸化翻译后修饰机制之间存在串扰。











































 京公网安备 11010802027423号
京公网安备 11010802027423号