当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
I2-Promoted [4 + 2] cycloaddition of in situ generated azoalkenes with enaminones: facile and efficient synthesis of 1,4-dihydropyridazines and pyridazines
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-10-29 , DOI: 10.1039/d0ob01958e Jiajun Feng 1 , Tiantong He 1 , Yuxing Xie 2 , Yang Yu 3 , Jonathan B Baell 4 , Fei Huang 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-10-29 , DOI: 10.1039/d0ob01958e Jiajun Feng 1 , Tiantong He 1 , Yuxing Xie 2 , Yang Yu 3 , Jonathan B Baell 4 , Fei Huang 5
Affiliation
|
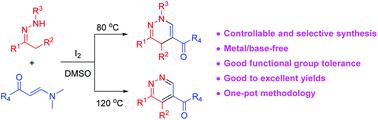
A facile and efficient strategy for the synthesis of 1,4-dihydropyridazines and pyridazines through I2-promoted [4 + 2] cycloaddition of in situ generated azoalkenes with enaminones has been developed. The switch in selectivity is attributed to the judicious choice of different reaction temperatures. The key features of this work include controllable and selective synthesis, good functional group tolerance, good to excellent reaction yields, metal/base-free conditions, and also applicability to one-pot methodology.
中文翻译:

I2-促进 [4 + 2] 原位生成的偶氮烯烃与烯胺酮的环加成:1,4-二氢哒嗪和哒嗪的简便有效合成
已经开发了一种通过 I 2 -促进的 [4 + 2]原位生成的偶氮烯烃与烯胺酮环加成来合成 1,4-二氢哒嗪和哒嗪的简便有效的策略。选择性的转换归因于对不同反应温度的明智选择。这项工作的主要特点包括可控和选择性合成、良好的官能团耐受性、良好的反应收率、无金属/碱基条件以及一锅法的适用性。
更新日期:2020-11-12
中文翻译:

I2-促进 [4 + 2] 原位生成的偶氮烯烃与烯胺酮的环加成:1,4-二氢哒嗪和哒嗪的简便有效合成
已经开发了一种通过 I 2 -促进的 [4 + 2]原位生成的偶氮烯烃与烯胺酮环加成来合成 1,4-二氢哒嗪和哒嗪的简便有效的策略。选择性的转换归因于对不同反应温度的明智选择。这项工作的主要特点包括可控和选择性合成、良好的官能团耐受性、良好的反应收率、无金属/碱基条件以及一锅法的适用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号