当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A low-temperature oxyl transfer to carbon monoxide from the ZnII–oxyl site in a zeolite catalyst
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-10-28 , DOI: 10.1039/d0qi01112f Akira Oda 1, 2, 3, 4, 5 , Jun Kumagai 4, 6, 7, 8 , Takahiro Ohkubo 4, 5, 9, 10, 11 , Yasushige Kuroda 4, 5, 9, 10, 11
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-10-28 , DOI: 10.1039/d0qi01112f Akira Oda 1, 2, 3, 4, 5 , Jun Kumagai 4, 6, 7, 8 , Takahiro Ohkubo 4, 5, 9, 10, 11 , Yasushige Kuroda 4, 5, 9, 10, 11
Affiliation
|
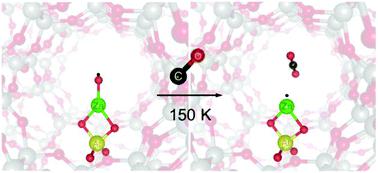
An atomic O radical anion bound to a metal ion (metal–oxyl) is a key intermediate in a variety of oxidative reactions. Understanding its structure–reactivity relationship is highly desirable for the rational design of challenging oxidative transformation processes. However, due to the difficulty of analysis, even the identification of an oxyl is a challenging subject especially in the research field of heterogeneous catalysts. Here, we report for the first time a low-temperature oxyl transfer to CO from the ZnII–oxyl bond isolated in a zeolite catalyst. Zeolite matrix isolation of this novel ZnII–oxyl bond allows us to observe the unique spectroscopic probes of the oxyl: a vibronically-resolved spectrum and ESR signatures. Using the oxyl-selective spectroscopic probes, we successfully demonstrated that the ZnII–oxyl bond has the capability of transferring the oxyl to CO even at 150 K with the generation of a single ZnI˙ species. The superhyperfine interaction of the ZnI˙ species with the framework Al atom, observed during the oxyl-transfer reaction, provided direct experimental evidence that the oxyl-functionality emerged at the framework Al site. DFT calculations showed that the ZnII–oxyl bond, which is constrained by the zeolite lattice ligation, acts as a superior electron donor toward CO at the rate-determining step of the oxyl-transfer reaction and effectively reduces the barrier to be <5 kJ mol−1. Based on the results obtained in the present study as well as the previous work, we further deepen the understanding of why the abnormal ZnII–oxyl bond having exceptional reactivities is formed by the zeolite lattice ligation.
中文翻译:

低温氧基从沸石催化剂中的ZnII–氧基位置转移到一氧化碳
与金属离子(金属-氧基)结合的原子O自由基阴离子是各种氧化反应的关键中间体。对于具有挑战性的氧化转化过程的合理设计,非常需要了解其结构-反应性关系。然而,由于分析的困难,即使是羟基的鉴定也是一个挑战性的课题,特别是在非均相催化剂的研究领域中。在这里,我们首次报道了从沸石催化剂中分离的Zn II-羟基键向CO的低温羟基转移。新型Zn II的沸石基质分离-oxyl键使我们能够观察到oxyl的独特光谱探针:通过纤维分辨的光谱和ESR标记。使用氧基选择性光谱探针,我们成功地证明了锌II -1-氧基键具有即使在150 K的一个单一的Zn的生成的氧基转移到CO的能力我˙物种。中的Zn的超超相互作用我˙与骨架Al原子时,烃氧基转移反应过程中观察到的物种,提供直接的实验证据表明,氧基功能出现在骨架Al位点。DFT计算表明,Zn II受沸石晶格连接限制的–oxyl键在氧基转移反应的速率确定步骤中作为CO的优良电子供体,有效地将势垒降低至<5 kJ mol -1。根据本研究以及先前的研究结果,我们进一步加深了对为什么通过沸石晶格结扎形成具有异常反应性的异常Zn II-羟基键的理解。
更新日期:2020-11-12
中文翻译:

低温氧基从沸石催化剂中的ZnII–氧基位置转移到一氧化碳
与金属离子(金属-氧基)结合的原子O自由基阴离子是各种氧化反应的关键中间体。对于具有挑战性的氧化转化过程的合理设计,非常需要了解其结构-反应性关系。然而,由于分析的困难,即使是羟基的鉴定也是一个挑战性的课题,特别是在非均相催化剂的研究领域中。在这里,我们首次报道了从沸石催化剂中分离的Zn II-羟基键向CO的低温羟基转移。新型Zn II的沸石基质分离-oxyl键使我们能够观察到oxyl的独特光谱探针:通过纤维分辨的光谱和ESR标记。使用氧基选择性光谱探针,我们成功地证明了锌II -1-氧基键具有即使在150 K的一个单一的Zn的生成的氧基转移到CO的能力我˙物种。中的Zn的超超相互作用我˙与骨架Al原子时,烃氧基转移反应过程中观察到的物种,提供直接的实验证据表明,氧基功能出现在骨架Al位点。DFT计算表明,Zn II受沸石晶格连接限制的–oxyl键在氧基转移反应的速率确定步骤中作为CO的优良电子供体,有效地将势垒降低至<5 kJ mol -1。根据本研究以及先前的研究结果,我们进一步加深了对为什么通过沸石晶格结扎形成具有异常反应性的异常Zn II-羟基键的理解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号