Current Organic Chemistry ( IF 1.7 ) Pub Date : 2020-08-31 , DOI: 10.2174/1385272824999200802181634 Marjan Mollazadeh 1 , Maryam Mohammadi-Khanaposhtani 2 , Yousef Valizadeh 3 , Afsaneh Zonouzi 1 , Mohammad Ali Faramarzi 4 , Parisa Hariri 4 , Mahmood Biglar 3 , Bagher Larijani 3 , Haleh Hamedifar 5 , Mohammad Mahdavi 3 , Nima Sepehri 6
|
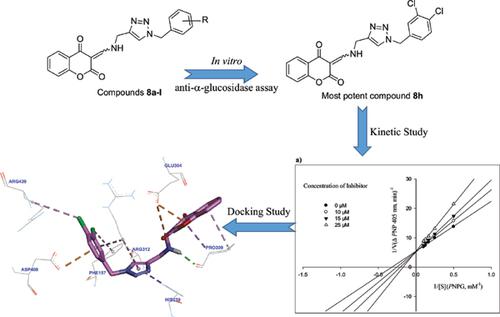
In this study, a novel series of 2,4-dioxochroman-1,2,3-triazole hybrids 8a-l was synthesized by click reaction. These compounds were screened against α-glucosidase through in vitro and in silico evaluations. All the synthesized hybrids exhibited excellent α-glucosidase inhibition in comparison to standard drug acarbose. Representatively, 3-((((1-(3,4-dichlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methylene)chroman-2,4- dione 8h with IC50 = 20.1 ± 1.5 μM against α-glucosidase, was 37-times more potent than acarbose. Enzyme kinetic study revealed that compound 8h was a competitive inhibitor against α-glucosidase. In silico docking study on chloro derivatives 8h, 8g, and 8i were also performed in the active site of α -glucosidase. Evaluations on obtained interaction modes and binding energies of these compounds confirmed the results obtained through in vitro α-glucosidase inhibition.
中文翻译:

与1,2,3-三唑衍生物作为新的α-葡萄糖苷酶抑制剂的2,4-二氧杂色满部分:合成,体外生物学评估和对接研究
在这项研究中,通过点击反应合成了一系列新的2,4-二氧杂色满-1,2,3-三唑杂化物8a-1。通过体外和计算机评估,筛选了针对α-葡萄糖苷酶的这些化合物。与标准药物阿卡波糖相比,所有合成的杂种均表现出优异的α-葡萄糖苷酶抑制作用。代表性地,3-(((((1-(3,4-二氯苄基)-1H-1,2,3-三唑-4-基)甲基)氨基)亚甲基)chroman-2,4-二酮8h,IC50 = 20.1抗α-葡萄糖苷酶的±1.5μM的效力是阿卡波糖的37倍。酶动力学研究表明,化合物8h是抗α-葡萄糖苷酶的竞争性抑制剂。在计算机对接研究中,还在α-葡萄糖苷酶的活性位点上对氯衍生物8h,8g和8i进行了研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号