当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Connecting Longitudinal and Transverse Relaxation Rates in Live-Cell NMR
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcb.0c08274 Sarah Leeb 1 , Fan Yang 1 , Mikael Oliveberg 1 , Jens Danielsson 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-12 , DOI: 10.1021/acs.jpcb.0c08274 Sarah Leeb 1 , Fan Yang 1 , Mikael Oliveberg 1 , Jens Danielsson 1
Affiliation
|
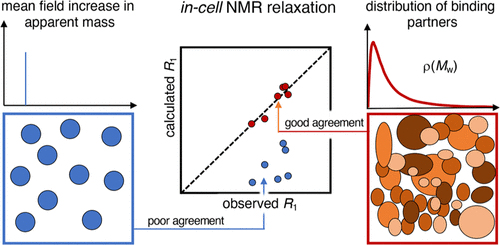
In the cytosolic environment, protein crowding and Brownian motions result in numerous transient encounters. Each such encounter event increases the apparent size of the interacting molecules, leading to slower rotational tumbling. The extent of transient protein complexes formed in live cells can conveniently be quantified by an apparent viscosity, based on NMR-detected spin-relaxation measurements, that is, the longitudinal (T1) and transverse (T2) relaxation. From combined analysis of three different proteins and surface mutations thereof, we find that T2 implies significantly higher apparent viscosity than T1. At first sight, the effect on T1 and T2 seems thus nonunifiable, consistent with previous reports on other proteins. We show here that the T1 and T2 deviation is actually not a inconsistency but an expected feature of a system with fast exchange between free monomers and transient complexes. In this case, the deviation is basically reconciled by a model with fast exchange between the free-tumbling reporter protein and a transient complex with a uniform 143 kDa partner. The analysis is then taken one step further by accounting for the fact that the cytosolic content is by no means uniform but comprises a wide range of molecular sizes. Integrating over the complete size distribution of the cytosolic interaction ensemble enables us to predict both T1 and T2 from a single binding model. The result yields a bound population for each protein variant and provides a quantification of the transient interactions. We finally extend the approach to obtain a correction term for the shape of a database-derived mass distribution of the interactome in the mammalian cytosol, in good accord with the existing data of the cellular composition.
中文翻译:

连接活细胞 NMR 中的纵向和横向弛豫率
在细胞质环境中,蛋白质拥挤和布朗运动导致大量短暂的相遇。每一次这样的相遇事件都会增加相互作用分子的表观尺寸,导致更慢的旋转翻滚。活细胞中形成的瞬时蛋白复合物的程度可以方便地通过表观粘度进行量化,基于NMR检测的自旋弛豫测量,即纵向( T 1 )和横向( T 2 )弛豫。通过对三种不同蛋白质及其表面突变的组合分析,我们发现T 2意味着比T 1显着更高的表观粘度。乍一看,对T 1和T 2的影响似乎无法统一,这与之前对其他蛋白质的报道一致。我们在这里表明, T 1和T 2偏差实际上不是不一致,而是自由单体和瞬态复合物之间快速交换的系统的预期特征。在这种情况下,偏差基本上是通过自由翻滚报告蛋白和具有统一 143 kDa 伴侣的瞬时复合物之间快速交换的模型来协调的。然后,通过考虑细胞溶质含量绝不是均匀的而是包含多种分子大小这一事实,进一步进行分析。整合胞质相互作用整体的完整尺寸分布使我们能够从单个结合模型预测T 1和T 2 。 结果产生了每种蛋白质变体的结合群体,并提供了瞬时相互作用的量化。最后,我们扩展了该方法,以获得哺乳动物细胞质中相互作用组的数据库衍生质量分布形状的校正项,与细胞组成的现有数据非常一致。
更新日期:2020-11-25
中文翻译:

连接活细胞 NMR 中的纵向和横向弛豫率
在细胞质环境中,蛋白质拥挤和布朗运动导致大量短暂的相遇。每一次这样的相遇事件都会增加相互作用分子的表观尺寸,导致更慢的旋转翻滚。活细胞中形成的瞬时蛋白复合物的程度可以方便地通过表观粘度进行量化,基于NMR检测的自旋弛豫测量,即纵向( T 1 )和横向( T 2 )弛豫。通过对三种不同蛋白质及其表面突变的组合分析,我们发现T 2意味着比T 1显着更高的表观粘度。乍一看,对T 1和T 2的影响似乎无法统一,这与之前对其他蛋白质的报道一致。我们在这里表明, T 1和T 2偏差实际上不是不一致,而是自由单体和瞬态复合物之间快速交换的系统的预期特征。在这种情况下,偏差基本上是通过自由翻滚报告蛋白和具有统一 143 kDa 伴侣的瞬时复合物之间快速交换的模型来协调的。然后,通过考虑细胞溶质含量绝不是均匀的而是包含多种分子大小这一事实,进一步进行分析。整合胞质相互作用整体的完整尺寸分布使我们能够从单个结合模型预测T 1和T 2 。 结果产生了每种蛋白质变体的结合群体,并提供了瞬时相互作用的量化。最后,我们扩展了该方法,以获得哺乳动物细胞质中相互作用组的数据库衍生质量分布形状的校正项,与细胞组成的现有数据非常一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号