当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Creating Red Light-Switchable Protein Dimerization Systems as Genetically Encoded Actuators with High Specificity
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-12 , DOI: 10.1021/acssynbio.0c00397 Zhimin Huang 1 , Zengpeng Li 1, 2 , Xiao Zhang 1 , Shoukai Kang 1 , Runze Dong 1 , Li Sun 1 , Xiaonan Fu 1 , David Vaisar 1 , Kurumi Watanabe 1 , Liangcai Gu 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-12 , DOI: 10.1021/acssynbio.0c00397 Zhimin Huang 1 , Zengpeng Li 1, 2 , Xiao Zhang 1 , Shoukai Kang 1 , Runze Dong 1 , Li Sun 1 , Xiaonan Fu 1 , David Vaisar 1 , Kurumi Watanabe 1 , Liangcai Gu 1
Affiliation
|
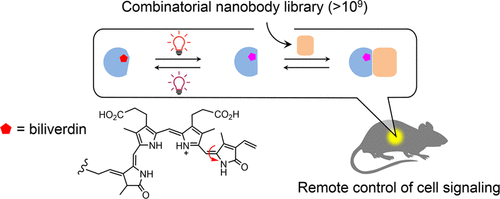
Protein dimerization systems controlled by red light with increased tissue penetration depth are a highly needed tool for clinical applications such as cell and gene therapies. However, mammalian applications of existing red light-induced dimerization systems are hampered by limitations of their two components: a photosensory protein (or photoreceptor) which often requires a mammalian exogenous chromophore and a naturally occurring photoreceptor binding protein typically having a complex structure and nonideal binding properties. Here, we introduce an efficient, generalizable method (COMBINES-LID) for creating highly specific, reversible light-induced heterodimerization systems independent of any existing binders to a photoreceptor. It involves a two-step binder screen (phage display and yeast two-hybrid) of a combinatorial nanobody library to obtain binders that selectively engage a light-activated form of a photoswitchable protein or domain not the dark form. Proof-of-principle was provided by engineering nanobody-based, red light-induced dimerization (nanoReD) systems comprising a truncated bacterial phytochrome sensory module using a mammalian endogenous chromophore, biliverdin, and light-form specific nanobodies. Selected nanoReD systems were biochemically characterized, exhibiting low dark activity and high induction specificity, and further demonstrated for the reversible control of protein translocation and activation of gene expression in mice. Overall, COMBINES-LID opens new opportunities for creating genetically encoded actuators for the optical manipulation of biological processes.
中文翻译:

创建具有高特异性的红光可切换蛋白质二聚系统作为遗传编码的致动器
由红光控制并具有增加的组织穿透深度的蛋白质二聚化系统是临床应用(如细胞和基因疗法)急需的工具。然而,现有的红光诱导的二聚化系统在哺乳动物中的应用受到以下两个方面的限制:通常需要哺乳动物外源发色团的光敏蛋白(或感光体)和通常具有复杂结构和非理想结合的天然存在的感光体结合蛋白属性。在这里,我们介绍一种有效的,可推广使用的方法(COMBINES-LID),用于创建高度特异性,可逆的光诱导异二聚系统,该系统独立于感光体的任何现有粘合剂。它涉及组合纳米抗体文库的两步结合剂筛选(噬菌体展示和酵母双杂交),以获得能选择性地与光激活形式的光转换蛋白质或结构域而非暗形式结合的结合剂。通过使用基于哺乳动物内源性发色团,胆绿素和轻型特异性纳米抗体的工程化的基于纳米抗体的,红光诱导的二聚化(nanoReD)系统提供原理证明,该系统包含截短的细菌植物色素感觉模块。选定的nanoReD系统经过生物化学表征,表现出较低的暗活性和高的诱导特异性,并进一步证明了可逆性控制蛋白质转运和激活小鼠基因表达。总体,
更新日期:2020-12-18
中文翻译:

创建具有高特异性的红光可切换蛋白质二聚系统作为遗传编码的致动器
由红光控制并具有增加的组织穿透深度的蛋白质二聚化系统是临床应用(如细胞和基因疗法)急需的工具。然而,现有的红光诱导的二聚化系统在哺乳动物中的应用受到以下两个方面的限制:通常需要哺乳动物外源发色团的光敏蛋白(或感光体)和通常具有复杂结构和非理想结合的天然存在的感光体结合蛋白属性。在这里,我们介绍一种有效的,可推广使用的方法(COMBINES-LID),用于创建高度特异性,可逆的光诱导异二聚系统,该系统独立于感光体的任何现有粘合剂。它涉及组合纳米抗体文库的两步结合剂筛选(噬菌体展示和酵母双杂交),以获得能选择性地与光激活形式的光转换蛋白质或结构域而非暗形式结合的结合剂。通过使用基于哺乳动物内源性发色团,胆绿素和轻型特异性纳米抗体的工程化的基于纳米抗体的,红光诱导的二聚化(nanoReD)系统提供原理证明,该系统包含截短的细菌植物色素感觉模块。选定的nanoReD系统经过生物化学表征,表现出较低的暗活性和高的诱导特异性,并进一步证明了可逆性控制蛋白质转运和激活小鼠基因表达。总体,











































 京公网安备 11010802027423号
京公网安备 11010802027423号